News and Events
New Strategy Shows Promise Against Deadly Breast Cancer in the Brain
A new NIH study points to a promising strategy for treating aggressive breast cancer that spreads to the brain, a complication with few effective options. Learn how blocking a key brain cell survival pathway could open the door to future therapies.
Read MoreResearchers develop new method to estimate cell-type-specific information from bulk tumor data
CCR researchers have established a way to extract cell-type-specific expression information from bulk gene expression data of a tumor sample. Their computational approach, which outperforms comparable state-of-the-art methods, evaluates thousands of tumor samples with minimal cost.
Read MoreBreast cancer stem cells become aggressive upon contact with macrophages
New findings suggest that breast cancer cells gain stem-like characteristics of invasiveness, aggressiveness and drug resistance upon contact with macrophages, specialized white blood cells in the immune system.
Read MoreA high-fiber diet may improve the response of melanoma patients to immunotherapy
A new study co-led by Giorgio Trinchieri, M.D., chief of the Laboratory of Integrative Cancer Immunology, found that a diet rich in fiber may help some people being treated for melanoma respond to immunotherapy treatment by influencing the gut microbiome. The new findings come from an analysis of people with melanoma and mouse models of the disease.
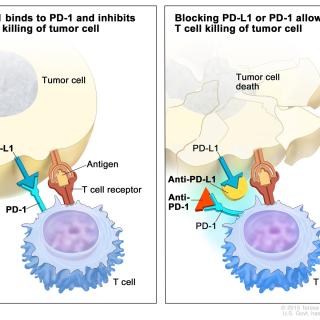
Read MoreCell surface molecule could predict how patients respond to immunotherapy
A new study finds that some immunotherapy drugs are less likely to control cancer in patients who carry HLA-A*03, a cell surface molecule that is involved in immune system responses. This work, which was featured in NCI's Cancer Currents, reveals that HLA-A*03 is a potential biomarker for patient prognosis following certain immunotherapy treatments.
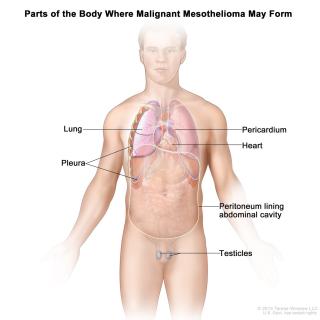
Read MoreClinical trial researches drug combination with immunotherapy for mesothelioma
Mesothelioma is a rare, fast-growing cancer that forms in membranes that surround and protect the heart, lungs and abdomen. The cells of malignant mesothelioma express large amounts of mesothelin, a cancerous protein. Investigators are researching a mesothelin-targeting drug in combination with immunotherapy.
Read MoreClinical trial targets cell surface protein GPC3 to treat advanced hepatocellular carcinoma
Tim F. Greten, M.D., Deputy Chief of the Thoracic and GI Malignancies Branch, is leading a study of advanced hepatocellular carcinoma, a common type of liver cancer with a poor prognosis. The research uses CAR T-cell therapy to target GPC3-positive tumor cells while avoiding healthy tissue.
Read MoreCCR researchers use immunogenetic profiling to identify targets for numerous pediatric cancers
Researchers analyzed over 700 pediatric tumor samples and cell lines, creating one of the largest profile datasets of gene expression and immune response in solid pediatric tumors to date. Their work reveals that a germline developmental protein called PRAME is a potential cancer target.
Read MoreAnish Thomas explains the challenges and goals of his small cell lung cancer research
Anish Thomas, M.B.B.S., M.D., Lasker Clinical Research Scholar in the Developmental Therapeutics Branch, studies small cell lung cancer (SCLC). For Lung Cancer Awareness Month, Thomas describes his current research interests and his goals to help improve the lives of patients with SCLC – the most lethal type of lung cancer.
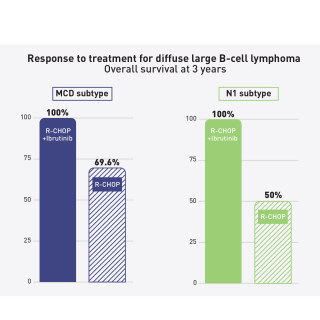
Read MoreIbrutinib improves survival for younger people with diffuse large B-cell lymphoma
New evidence suggests that adding the drug ibrutinib to a standard chemotherapy regimen can improve how long some younger people with a specific form of diffuse large B-cell lymphoma live. The findings come from a new analysis led by Louis M. Staudt, M.D., Ph.D., Chief of the Lymphoid Malignancies Branch, of a previous phase III clinical trial.
Read MoreClinical trial studies breast cancer drug abemaciclib as a therapy for Kaposi sarcoma
Kaposi sarcoma (KS) is a rare cancer that causes patches of abnormal tissue to develop in different regions of the body, and lesions in the lungs, liver, or digestive tract can be life-threatening. Investigators are studying abemaciclib, a drug used for people with breast cancer, to see if it can positively impact those with KS.
Read More