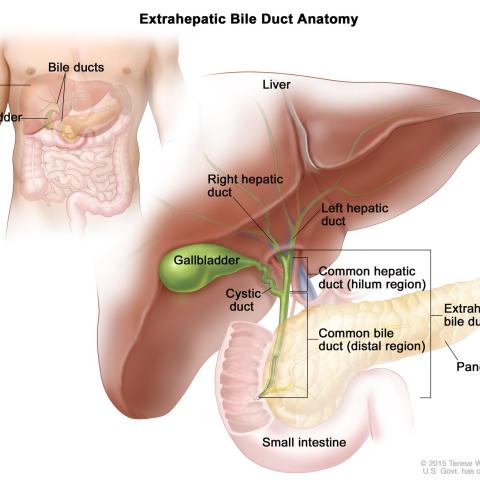
Anatomy of the extrahepatic bile ducts; drawing shows the liver, right and left hepatic ducts, gallbladder, cystic duct, common hepatic duct (hilum region), common bile duct (distal region), extrahepatic bile duct, pancreas, and small intestine. An inset shows the liver, bile ducts, and gallbladder.
Image credit: Terese Winslow
Adults with biliary tract cancer with a mutation of the KRAS gene may be eligible to participate in a clinical trial at the NIH Clinical Center.
Tim F. Greten, M.D., Deputy Chief of the Thoracic and GI Malignancies Branch, is leading a study of a drug combination that may prolong survival in adults with biliary tract cancer (BTC). BTC is a cancer that arises from the bile ducts that carry bile, a digestive fluid, through the liver. It’s a rare, but fast-growing cancer that often has an abnormal or mutated KRAS gene. There are few treatment options for BTC. Study participants will take trametinib and hydroxychloroquine (HCQ) tablets every day in 28-day cycles. Trametinib blocks the action of MEK, a signaling chemical that helps cells multiply and survive. However, cancer cells can get around trametinib’s MEK blockade by boosting a process called autophagy. Autophagy is the body’s way of eliminating damaged cells in order to generate new cells. HCQ inhibits autophagy so that cancer cells damaged by trametinib can’t regenerate themselves. In pre-clinical studies, the combination of trametinib and HCQ showed ability to shrink tumors. Researchers want to study how this drug combination may be effective in BTC with mutated KRAS gene.
Clinicaltrials.gov identifier: NCT04566133
NCI Protocol ID: NCI-20-C-0152
Official Title: Combination of Trametinib (MEK Inhibitor) and Hydroxychloroquine (HCQ) (Autophagy Inhibitor) in Patients with KRAS Mutation Refractory Bile Tract Carcinoma (BTC)
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.