Micrograph of small cell carcinoma.
Photo credit: OGPhoto from Getty Images Signature.
Adults with relapsed small cell lung cancer or high-grade neuroendocrine cancers may be eligible to participate in a new clinical trial at the NIH Clinical Center.
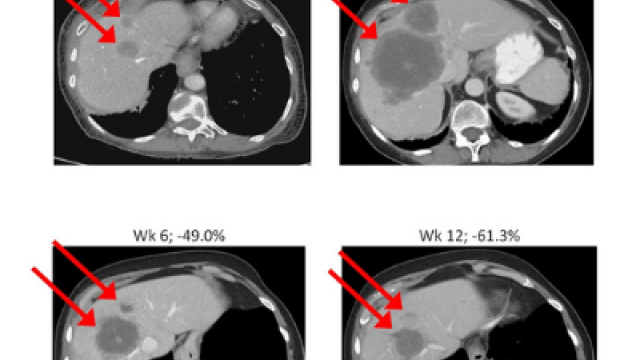
Anish Thomas, M.B.B.S., M.D., Lasker Clinical Research Scholar in the Developmental Therapeutics Branch (DTB), and Jaydira Del Rivero, M.D., Assistant Research Physician in DTB, are heading a trial of a treatment for small cell lung cancer (SCLC) or high-grade neuroendocrine cancers. SCLC and high-grade neuroendocrine cancers typically respond to initial chemotherapy but come back quickly and are resistant to additional chemotherapies. Chemotherapy-resistant cancer cells depend on a protein called ATR for DNA repair. Prior work from DTB investigators has shown that targeting ATR can reverse chemotherapy resistance in the lab and in patients. In this study, participants will receive berzosertib, an ATR inhibitor, in combination with lurbinectedin, a recently approved treatment for relapsed SCLC. The phase I part of the trial will find the highest dose of the drug combination that is safe. Phase II will assess how effectively the drug combination treats SCLC and high-grade neuroendocrine tumors.
Clinicaltrials.gov identifier: NCT04802174
NCI Protocol ID: NCI-00-0-176
Official Title: Lurbinectedin With Berzosertib, an ATR Kinase Inhibitor in Small Cell Cancers and High-Grade Neuroendocrine Cancers
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.