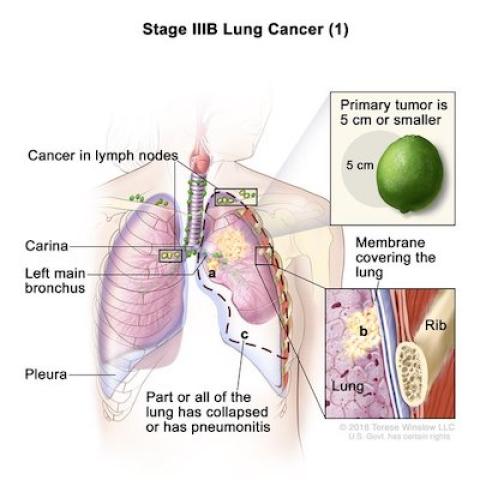
Lung Cancer, Stage IIIB (1)
Image source: NCI Visuals Online; Illustrator: Terese Winslow
Adults with epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer that has transformed to small cell lung cancer or a neuroendocrine tumor may be eligible to participate in a clinical trial at the NIH Clinical Center.
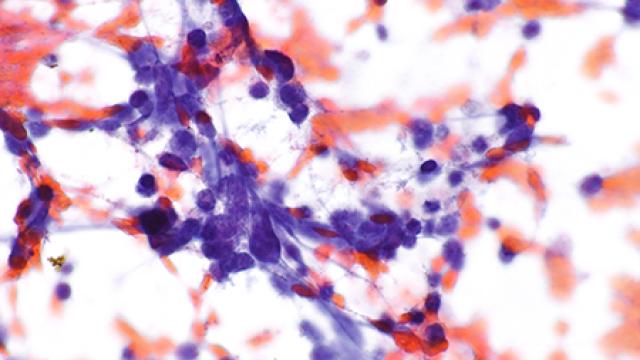
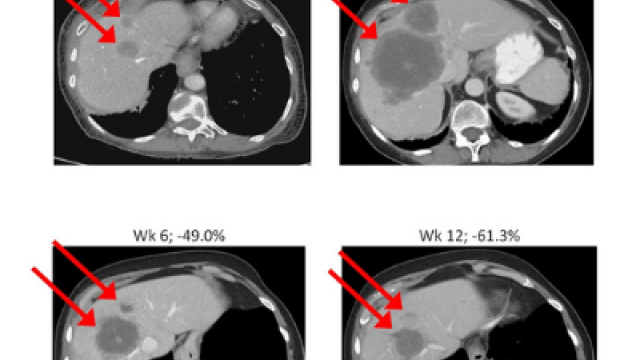
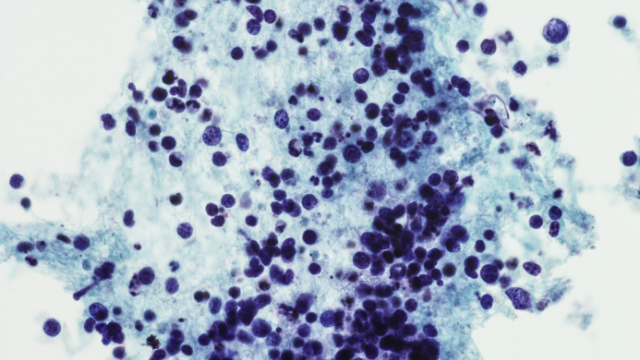
Non-small cell lung cancer with an EGFR mutation can become resistant to EGFR-targeted treatments by transforming into small cell lung cancer (SCLC), an aggressive cancer that has no standard treatment. Anish Thomas, M.B.B.S., M.D., NIH Lasker Clinical Research Scholar in the Developmental Therapeutics Branch, is leading a trial testing a drug combination to treat these kinds of tumors. Participants will get durvalumab which stops a protein on cancer cells from binding with a protein on immune cells known as T cells. This allows the T cells to do their job of attacking cancer cells. Participants will also take olaparib. Cancer cells rely on an enzyme called PARP to repair damage to their DNA and enable them to continue dividing and growing. Researchers want to see if the combination of durvalumab and olaparib can reduce the growth of small cell cancers that emerge under selection pressure from EGFR-targeted therapies.
Clinicaltrials.gov identifier: NCT04538378
NCI Protocol ID: NCI-20-C-0149
Official Title: Olaparib (LYNPARZA) Plus Durvalumab (IMFINZI) in EGFR-Mutated Adenocarcinomas That Transform to Small Cell Lung Cancer (SCLC) and Other Neuroendocrine Tumors
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.