Surgery Branch
Surgery Branch
About
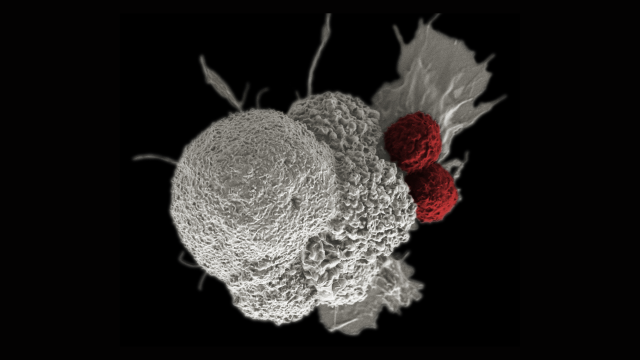
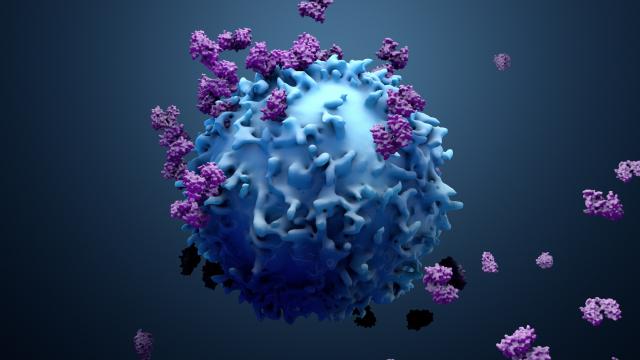
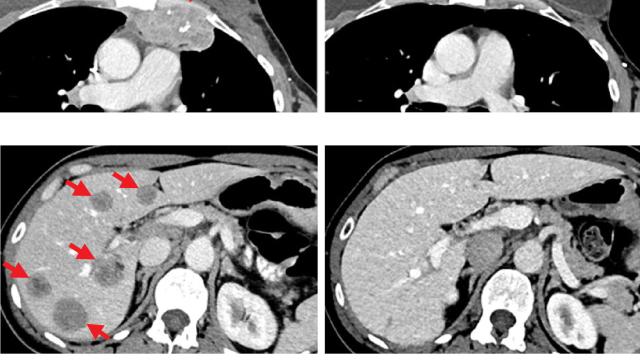
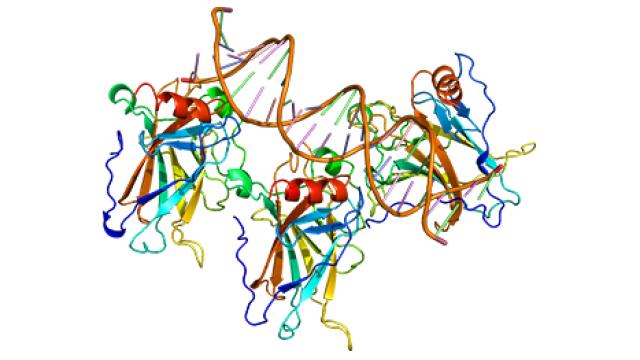
The Surgery Branch of the National Cancer Institute is a combined laboratory and clinical research unit devoted to the development of innovative cancer immunotherapies and their translation to the treatment of patients with cancer. Efforts run the gamut from basic studies of cancer immunology to the conduct of clinical immunotherapy trials for patients with metastatic cancer. The Surgery Branch was responsible for the development of interleukin-2 (IL-2), the first effective immunotherapy in humans, the development of cell transfer immunotherapies for melanoma and other solid cancers, the first insertion of foreign genes into humans and the first development of effective human cancer immunotherapies based on the genetic engineering of autologous lymphocytes with genes encoding anti-tumor T cell receptors or chimeric antigen receptors.
Examples of current laboratory research involve the identification of the optimal properties of lymphocytes capable of mediating anti-tumor effects in experimental models and in the human and the development of immunotherapies based on the identification of unique exomic mutations expressed by cancers that can be targeted by cell transfer therapy. Extensive programs are in place for the genetic modification of lymphocytes to target cancer-testis antigens. The Surgery Branch offers postdoctoral fellowships in cancer immunology and immunotherapy. Clinical fellowships are available to surgical residents who have completed at least two years of residency and are interested in a combined program of laboratory and clinical research in immunotherapy.
Interested in Our Clinical Trials?
Patients interested in our clinical trials should visit Frequently Asked Questions to learn more.
Clinical Trials
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
News
Contact
Contact Info
Center for Cancer Research National Cancer Institute
- Building 10, Room 3-3940
- Bethesda, MD 20892-1201
- 240-858-3080