CAR T-cell therapy homes in on a new target: neuroblastoma.
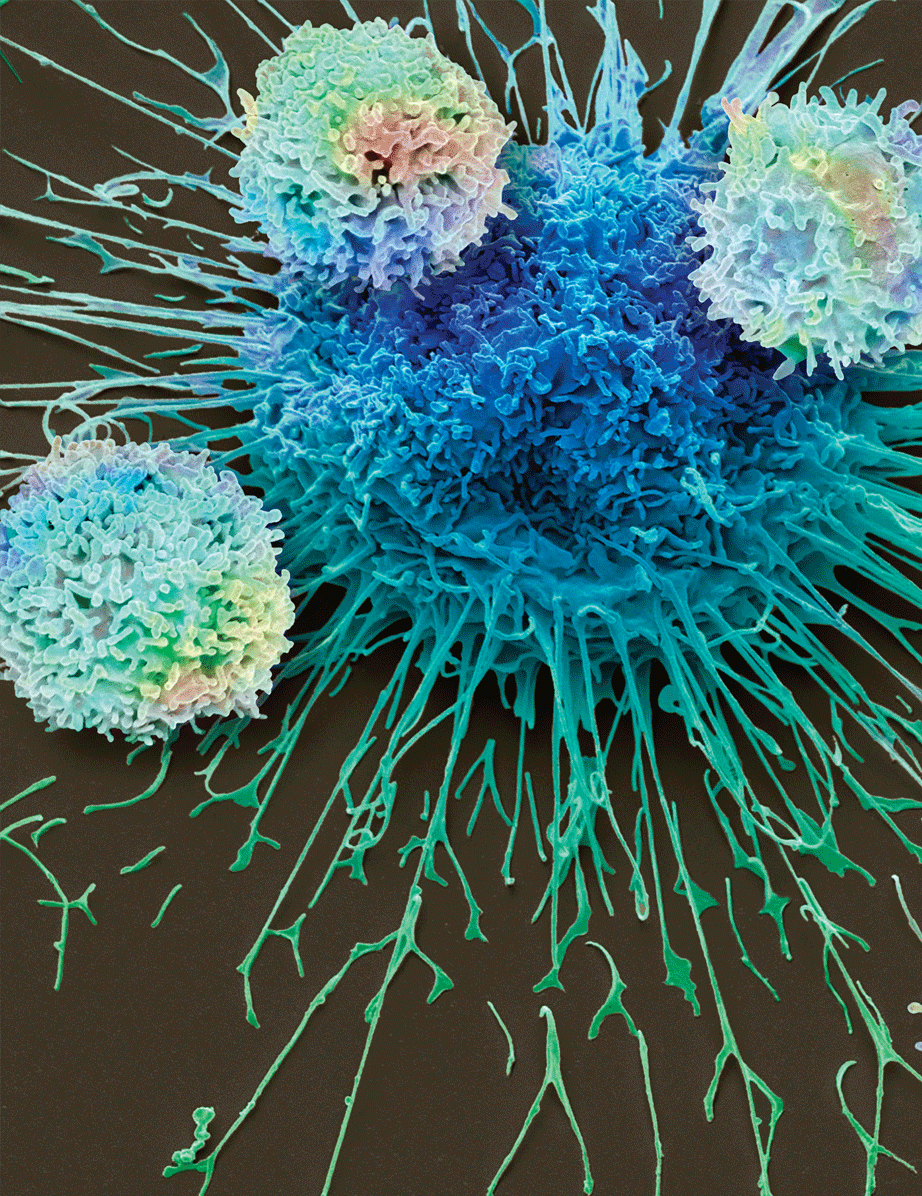
In this scanning electron microscopy image, CAR T cells attack a lung cancer cell, colored in blue at the center of the image. CAR T cells are immune cells that are artificially programmed to recognize unique proteins on the surface of cancer cells. Researchers discovered that CAR T cells can be programmed to fight neuroblastoma, an extremely rare and aggressive form of cancer that mainly affects children. Credit: Steve Gschmeissner/Science Photo Library
The emergence of chimeric antigen receptor (CAR) T-cell therapy has provided renewed hope for many patients with blood cancers who have not benefited from traditional therapies. However, ways to use this therapy for many other cancers have remained elusive — until now. Senior Investigator Mitchell Ho, Ph.D., and colleagues have discovered a way to target solid tumors through CAR T-cell therapy.
In CAR T-cell therapy, physicians program a patient’s immune cells to recognize unique proteins on the surface of cancer cells to help them better target and kill the cancer. The key to success, however, is to target a protein that is found only on the cancer cells, not on healthy ones. While CAR T cells can safely target and destroy lymphoma and leukemia blood cells without harming other organs in the body, researchers have struggled to identify tumor-specific proteins that can be used in CAR T-cell therapy to target solid cancers without harming healthy organs in the process.
Ho is in a unique position to solve this problem because he studies proteins that are only expressed in cancers. In 2014, Ho and postdoctoral fellow Nan Li, Ph.D., began to investigate one such protein, called GPC2. Early evidence showed that it is expressed in neuroblastoma, an extremely rare and notoriously difficult-to-treat form of cancer that mainly affects children.
As Li studied GPC2 and investigated its prevalence in healthy human tissues, she found that almost all the tissues were GPC2-negative, which made GPC2 an ideal candidate for CAR T-cell therapy with the potential to treat neuroblastoma. The team reported their finding in Proceedings of the National Academy of Sciences.
As a next step, the researchers sought to engineer a new type of CAR T cell capable of targeting GPC2. They identified an antibody called CT3, which is a protein that can bind to GPC2 but not to other targets. The researchers then modified T cells to express CT3, which helps the immune cells better target, bind to and kill GPC2-expressing cancer cells. When it came time to test their CAR T cells, their efforts proved fruitful.
“With only a single CAR T-cell treatment, we could see a dramatic effect,” says Ho. “In all preclinical models we looked at, our CAR T cells showed activity against neuroblastoma, leading to complete remission of tumors in mice.” The results were described in Cell Reports Medicine.
As a next step, the team is partnering with the Pediatric Oncology Branch to test this therapy in a first-in-human clinical trial led by Rosa Nguyen, M.D., Ph.D., a Physician-Scientist Early Investigator, with funding from the NCI Cancer MoonshotSM program. “If this therapy proves beneficial for patients, it would be a remarkable achievement in a short timeline,” Ho says, noting that the full spectrum of research on GPC2 CAR T cells — from basic science to a clinical trial — will be done by CCR.
Ho, Li and Nguyen believe that this new therapy could improve outcomes for patients with neuroblastoma and may even apply to other solid tumors.