Researchers identify a gene critical for embryonic development.
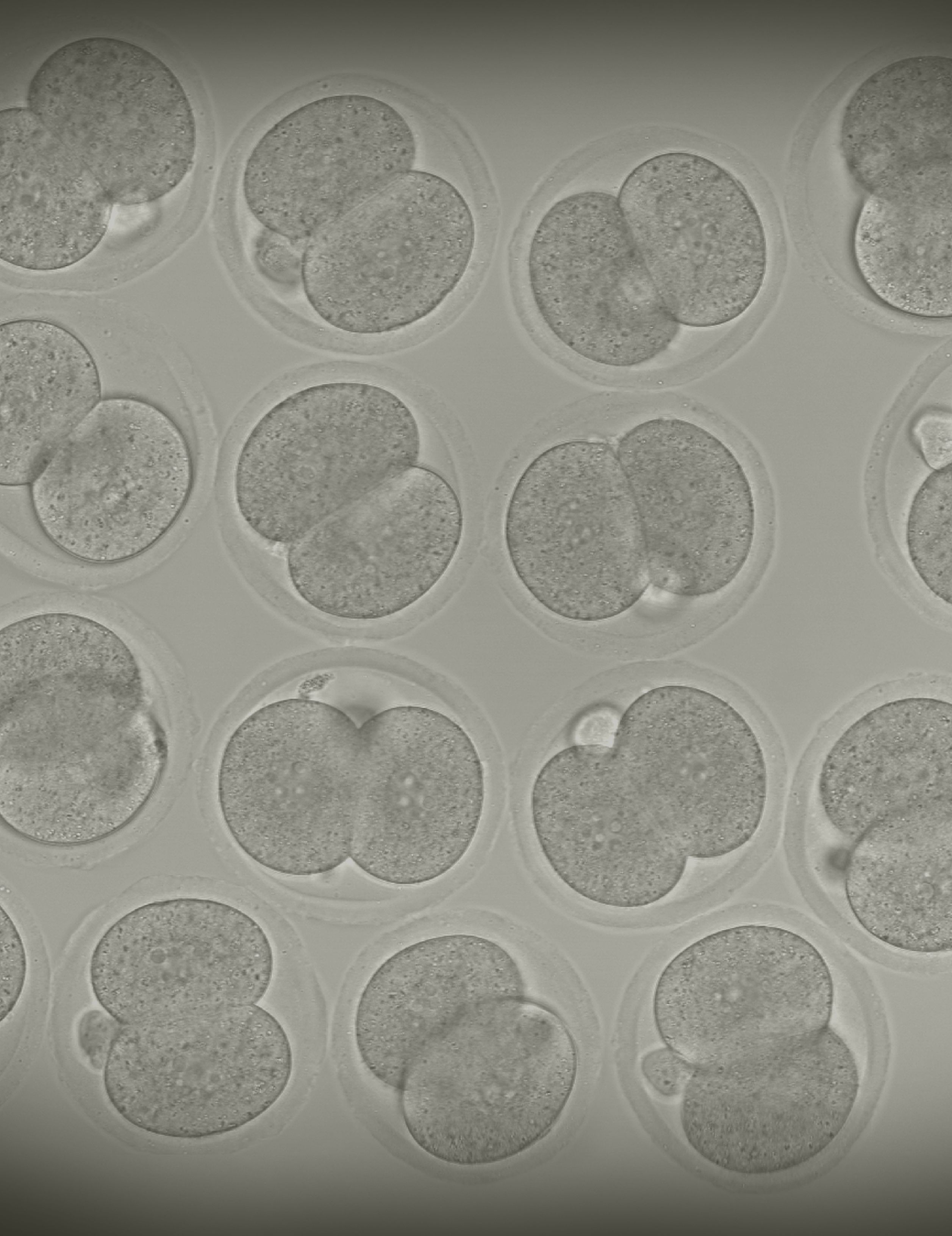
This image shows a collection of mouse embryos at the 2-cell stage similar to the ones used in this work. Changes in gene expression at this point are critical for the continued development of the embryos.
Credit: Paula Stein, NIEHS, NIH; SPGM, FNL, NCI, NIH; iStock
The first two cells that arise from a fertilized egg are totipotent, meaning they are able to become any type of cell in the body or in the structures required to support an embryo’s development, such as the placenta. For successful development to continue, these two cells must divide to make pluripotent cells which are then capable of making any cell in the body, but not in the placenta or other supporting structures. Pluripotent cells continue to divide and specialize as the embryo matures. Mammals from mice to humans are estimated to produce 200 to 300 types of cells that perform specific functions.
Cancer cells are notorious for being able to hijack genetic pathways that regulate cell development and specialization. By using genes in these pathways, they can revert to an earlier, more flexible stage that allows them to divide, mutate and adapt to new environments more easily, while evading cancer therapies. Understanding the regulatory pathways cancer cells use to re-program to these earlier states could lead to new treatment approaches.
For years, researchers have known that the Dux family of genes is responsible for regulating the expression of totipotent genes in mice, with similar genes identified in humans. However, these genes must be expressed only briefly before being turned off during the transition toward pluripotency. Any missteps in the process can lead to developmental issues or failure to form an embryo. In an important advance, published in Nature Genetics, researchers studying mice have identified a gene called Duxbl that acts as an off switch for Dux-induced genes, paving the way for healthy embryonic development.
“There was nothing known about Duxbl, and it turns out that it is essential,” explains Stadtman Tenure-Track Investigator Sergio Ruiz Macias, Ph.D., who led the research. “Without this gene, there is no organism.”
Ruiz Macias and Maria Vega-Sendino, Ph.D., a postdoctoral fellow in his lab, were curious to study the function of Duxbl given how it belongs to a family of genes activated during embryonic development and yet its specific function remained unknown.
They used microscopy, mass spectrometry and genomics to study embryonic stem cells and fertilized mouse eggs lacking the Duxbl gene. Ruiz Macias emphasizes that the work was highly technical and was facilitated through CCR’s intramural collaborations and investment in resources. In collaboration with Felipe F. Lüttmann and Johnny Kim, Ph.D., at the Max Planck Institute for Heart and Lung Research in Germany as well as colleagues at the NIH’s National Institute of Environmental Health Sciences in North Carolina, they showed that Dux-induced genes, including Duxbl, are triggered once fertilization takes place. But without Duxbl, these genes remain activated and actually block healthy embryonic development, revealing a repressive role for the protein produced by Duxbl on these genes. The effect is so strong that embryonic cells lacking DUXBL protein could not divide more than twice, making the embryo nonviable.
Ruiz Macias and his colleagues also identified two protein repressors, TRIM24 and TRIM33, that interact with the DUXBL protein. In future work, the researchers plan to study the repressive mechanisms of Duxbl in greater detail, digging deeper into the inner mechanisms behind this silencing gene.