New insights reveal how bone marrow cells impact the survival of plasma cells and their antibodies.
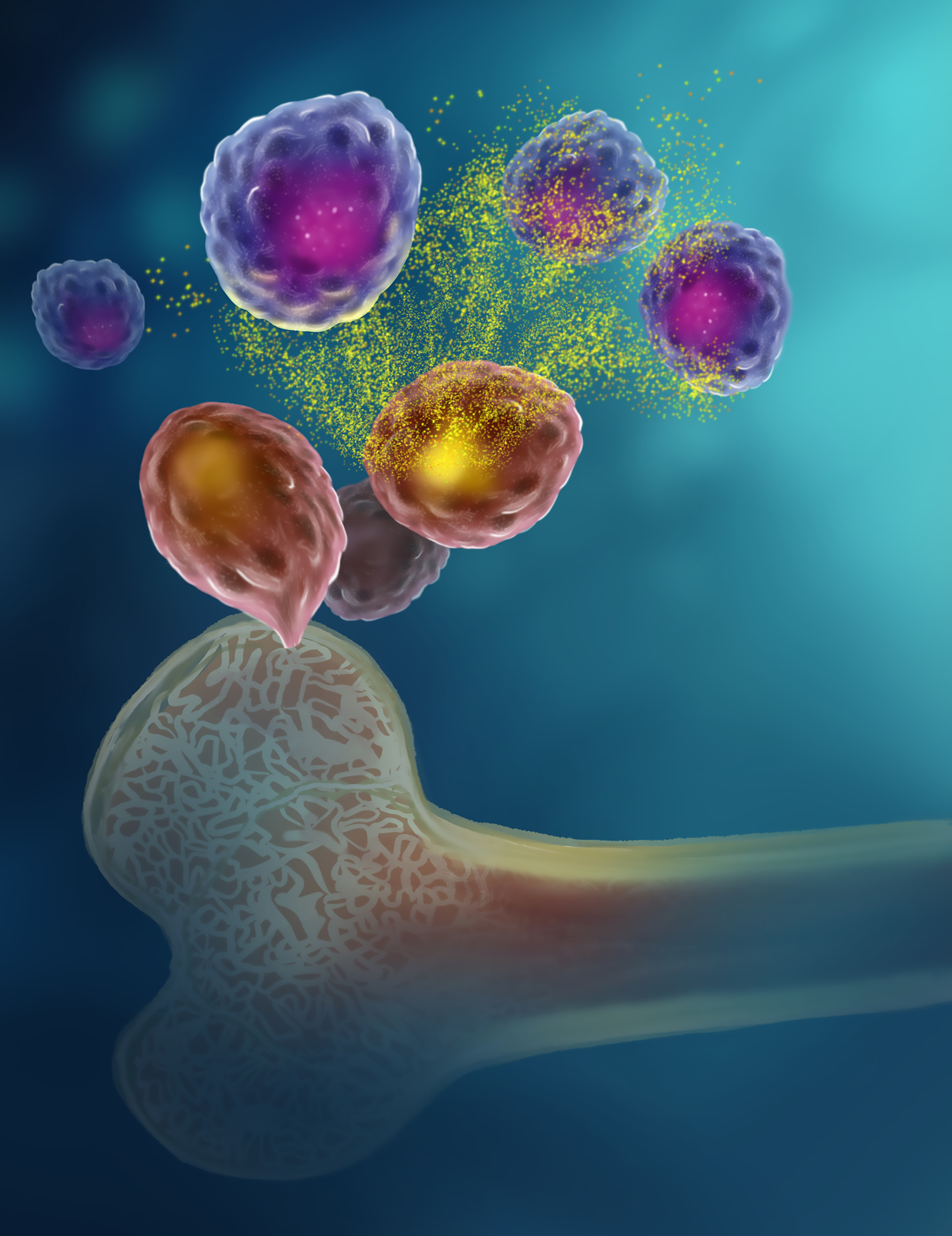
A team of researchers discovered an unexpected way that bone marrow cells and plasma cells are connected. This image illustrates the general findings: bone marrow cells (pink) release the energy molecule ATP (yellow), and a protein on the surface of plasma cells (purple) allows the cells, including long-lived plasma cells, to pick up and respond to the ATP.
Credit: SPGM, FNL, NCI, NIH
When a dentistry researcher encountered a curious protein produced by teeth and other bone-related cells, he approached CCR researchers to help him dig deeper to understand its function. Their unusual partnership led to the discovery that bone marrow cells play a critical role in regulating the immune system’s long-term memory via plasma cells.
Plasma cells are specialized immune cells that secrete antibodies, which are the immune proteins that recognize and attack harmful viruses and bacteria. A subset of plasma cells live longer than others, retaining valuable long-term memories of these pathogens after initial exposure. They live in the supportive microenvironment of the bone marrow, but how that environment impacts their lifespan has been poorly understood.
In 2007, Masaki Ishikawa, D.D.S., Ph.D., was at the NIH’s National Institute of Dental and Craniofacial Research where he studied a protein, called pannexin-3, which was highly expressed in teeth and in bone-related cells, but not other organs. Over time, he began to suspect it could influence the immune system.
Later, while at Tohoku University in Japan, Ishikawa reached out to Senior Investigator Avinash Bhandoola, M.B.B.S., Ph.D., who studies immune cell development. They then recruited David Allman, Ph.D., an expert on plasma cells at the University of Pennsylvania.
The researchers found that mice lacking the gene needed to produce pannexin-3 had reduced plasma cell numbers in bone marrow, as well as reduced antibody levels. Notably, pannexin-3 forms a channel in a cell’s surface membrane that allows it to release ATP, an energy molecule.
This prompted the team to explore whether pannexin-3 in bone cells was helping to supply life-sustaining signals in the form of ATP to plasma cells, which they confirmed. They also identified the P2RX4 receptor on plasma cells as responsible for sensing the ATP after it had been released through pannexin-3. Blocking P2RX4 in mice caused a significant decrease in bone marrow plasma cells and antibodies, including long-lived plasma cells, similar to eliminating pannexin-3.
This discovery, published in Nature, shows a novel and essential way in which bone marrow supports healthy sustenance of plasma cells, with clinical implications.
“We’re thinking this new knowledge should be useful for autoimmunity caused by antibodies,” Bhandoola explains.
Autoimmune diseases occur when a person’s immune system produces antibodies that mistakenly bind to their own tissues, called autoantibodies. In mice modelling the autoimmune disease lupus, the researchers found that blocking the P2RX4 channel on plasma cells decreased the animals’ autoantibody levels and lessened some of their lupus symptoms.
Along with implications for autoimmune disorders, Bhandoola believes these findings could provide new insights into the antibody protection gained from vaccines, eventually boosting their efficacy.
In addition, Bhandoola and Allman are now working with CCR colleagues to see if already existing drugs that inhibit P2RX4 can be valuable in treating multiple myeloma, a type of cancer that begins in plasma cells.
Bhandoola adds that this research — where Ishikawa was able to approach him and they were able to pursue a scientific curiosity without writing a grant — was made possible at CCR. “Masaki was able to follow an intuition,” he says. “I think it would have been very difficult to do anywhere else.”



