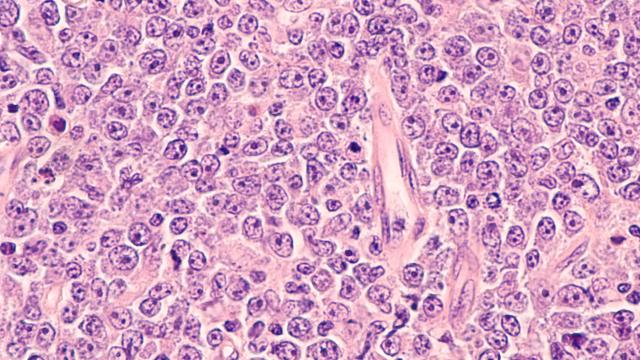
A novel drug combination likely cured more than a third of study participants of their lymphoma.
Lynda Flom had received standard treatments twice for lymphoma, starting in 2009, yet her cancer kept recurring. In 2017, she was in an exercise class in her hometown of Pittsburgh when she happened to strike up a conversation with an NIH researcher visiting from out of town. The new relationship prompted Lynda to seek treatment at NIH. When her lymphoma became very aggressive in 2018, NIH offered her the chance to participate in the ViPOR trial. “I was willing to give it a try, and I’m glad I did,” Lynda says. Although she had a bad reaction to the first dose of the ViPOR regimen, she says the tumor “melted like crazy” shortly after starting treatment. It has been just over six years since Lynda received the novel drug combination, and her lymphoma hasn’t returned. “It’s very nice to see those clean scans when I come back to NIH for checkups,” she says, noting that ViPOR offers another option for patients who don’t respond to standard therapy.
The colorful backdrop is a plot of responses to different doublet combinations of the ViPOR drugs in DLBCL cell lines. Creating the ViPOR treatment would not have been possible without close collaboration between researchers in CCR labs and clinical teams.
Credit: Photo courtesy of Lynda Flom; Melani, C., et al.; SPGM, FNL, NCI, NIH
A clinical trial evaluating a novel drug combination has yielded dramatic results for people with forms of aggressive lymphoma, with more than a third of patients seeing their lymphoma disappear completely and not return. The results were so beneficial that the research group behind the discovery is seeking to establish this drug combination — called ViPOR — as a standard treatment for some lymphoma subtypes.
The advance, described in the New England Journal of Medicine, is part of a multi-decade translational science endeavor by Senior Investigators Wyndham Wilson, M.D., Ph.D., and Louis Staudt, M.D., Ph.D., who led the clinical and laboratory work, respectively. The duo has been working together for years to identify lymphoma subtypes and test targeted therapies for each one.
Inspired by the way that different chemotherapy drugs are combined to launch a strong attack against cancer, the researchers sought to create a drug cocktail that targets specific pathways malignant lymphoma cells use for survival. They came up with the ViPOR regimen (venetoclax, ibrutinib, prednisone, obinutuzumab and lenalidomide) based on the hypothesis that targeting multiple critical survival pathways was necessary to achieve a cure.
When Staudt studied the effects of ViPOR on lymphoma cells in the laboratory, he observed the cancer cells dying in a matter of hours. “These cancer cells were quickly dead as a doornail,” he notes. “This was such a robust finding that I thought it would translate into clinical responses.”
Wilson and Staudt then partnered with Associate Research Physician Christopher Melani, M.D., and Senior Clinician Mark Roschewski, M.D., to lead a clinical trial investigating the effects of ViPOR in lymphoma patients whose cancer had persisted despite standard treatment.
A total of 50 patients with diffuse large B-cell lymphoma (DLBCL) were given ViPOR in a phase 1/2 trial. More than half of the study participants responded to therapy, with 38 percent showing no trace of lymphoma after just a few brief cycles of treatment with ViPOR. Notably, ViPOR was also effective in patients who had received CAR T-cell therapy for refractory lymphoma after treatment with chemotherapy. Melani notes that aggressive lymphomas often recur quickly if treatment proves ineffective, yet many patients in this study have been lymphoma-free for several years since being treated in the trial. “The patients who are still in remission after five or six years are very likely cured of their lymphoma,” he says, noting that seeing these results is very gratifying. While the drug combination involved some side effects, such as reduced white blood cell counts, they generally were not severe or worse than standard lymphoma therapies.
Louis M. Staudt, M.D., Ph.D.
Chief
Lymphoid Malignancies Branch
Wyndham Wilson, M.D., Ph.D.
Senior Investigator
Lymphoid Malignancies Branch
Most of the patients who experienced full remissions had certain molecularly defined lymphoma subtypes, specifically non-germinal center B-cell DLBCL or high-grade B-cell lymphoma with genetic rearrangements of the MYC and BCL2 genes. A larger follow-up clinical trial is now underway in multiple centers nationally to test ViPOR in more patients, as part of NCI-funded research.
Staudt emphasizes that this research could not have been done without long-term support from CCR and close collaborations where he could make discoveries in the lab and partner quickly with colleagues to test those findings in the clinic. “That's really remarkable and doesn't happen at a lot of other places,” Staudt says.