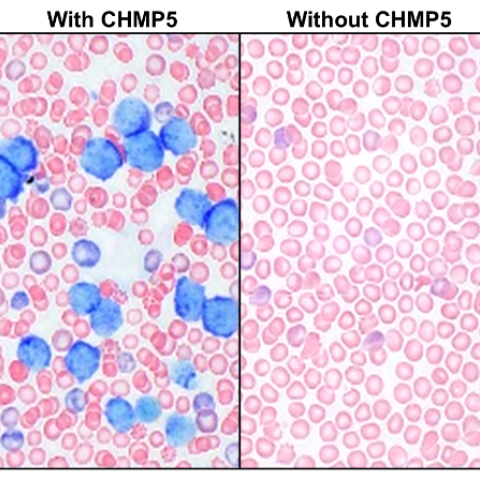
Blood from wildtype mice (left) showing blast-like T-ALL cells with characteristic blue nuclear staining; blood from mice in which CHMP5 is deficient in thymocytes (right) showing no evidence of T-ALL cells in the blood.
CCR researchers discovered a new role for the CHMP5 protein, which was previously thought to only regulate how cells divide or repair membranes. Using T-cell acute lymphoblastic leukemia (T-ALL) cells and animal models, the researchers, led by Stanley Adoro, Ph.D., Stadtman Tenure-Track Investigator in the Experimental Immunology Branch, and graduate student Katharine Umphred-Wilson, found that T-ALL cells depend on CHMP5 to promote transcription factors that initiate and sustain expression of cancer genes. The research was published May 3, 2025, in Nature Communications.
While researchers know that oncogenes, including those that cause T-ALL, can take control of the transcription machinery in cells to drive the expression of genes and proteins that help tumor cells proliferate and survive, they don’t know how they do this. This knowledge could help develop novel cancer therapies.
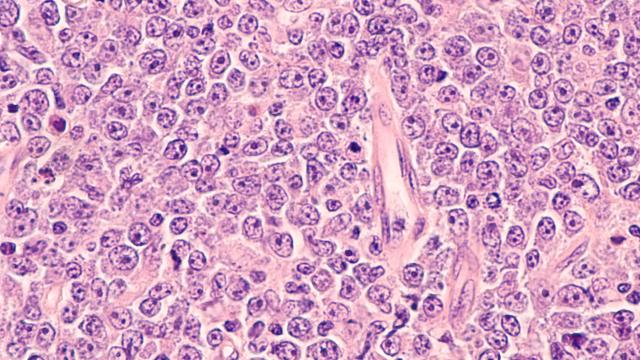
T-ALL is an aggressive blood cancer that arises in immature T cells, called thymocytes, during their differentiation into T lymphocytes, a type of white blood cell. The researchers found that CHMP5 levels were high in T-ALL cells compared to normal T lymphocytes and that the protein helps assemble two transcription factors, BRD4 and p300, which are notable for their high activity and regulation of cancer-specific genes. In contrast, depleting CHMP5 in T-ALL cells impaired cell proliferation and increased their sensitivity to chemotherapeutic agents. The researchers also found that depletion of CHMP5 prevented oncogenes from even initiating T-ALL disease.
This research advances the understanding of what causes T-ALL to arise, and because CHMP5 is highly expressed in other types of cancers, the researchers suspect it plays a similar role in these as well. While CHMP5 cannot currently be targeted, they believe that dual inhibition of BRD4 and p300 could be an effective strategy for killing T-ALL cells.
Citation: Umphred-Wilson, K., et al. Nat Commun. 2025 May 3;16(1):4133.
Writeup information provided by Stanley Adoro, Ph.D., and Katharine Umphred-Wilson.