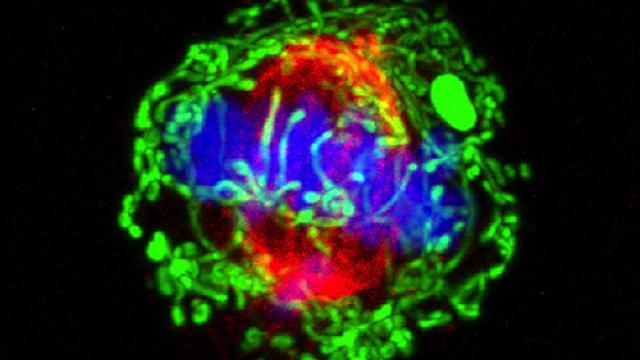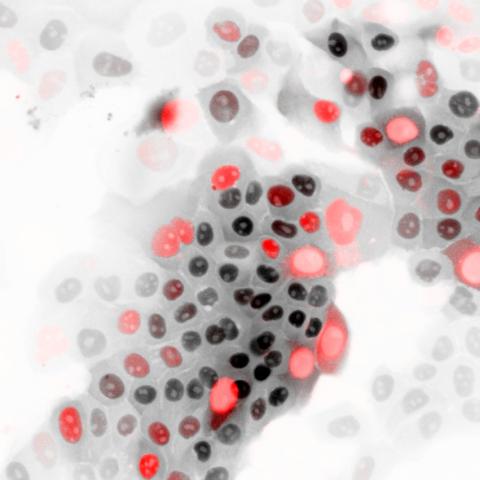
Cells showing the biosensor used in this study to test the activity of APC/C. Image credit: Cappell lab
A study led by CCR scientists has provided new information about the activity of an enzyme called anaphase-promoting complex/cyclosome (APC/C) during cell division. Previously, researchers believed that the enzyme remained active throughout the early parts of the cell cycle, but the team found that APC/C transiently turned on and off as cells begin the cell cycle. This discovery, published July 20, 2025, in Nature, could eventually be used to create treatments that target the enzyme and inhibit cancer cell proliferation. The study was led by Steven D. Cappell, Ph.D., Stadtman Investigator in the Laboratory of Cancer Biology and Genetics, and first author Debasish Paul, Ph.D., a postdoctoral fellow in Cappell’s lab.
Most cells in the body are not actively dividing — they are in an inactive state called quiescence. To repair wounds or stimulate the immune system when necessary, cells must begin to divide. This requires energy and new biomass, which cells can obtain through glycolysis, a process that uses glucose to create energy and cellular building blocks.
To prepare for division, cells also need to engage in several processes, including preparing DNA for replication, which is typically a mutually exclusive event with glycolysis and was previously thought to occur separately.
The researchers used time-lapse microscopy to watch as individual cells entered the cell cycle, and what they saw appeared to be a paradox. They noticed that the APC/C protein was involved in both producing biomass and preparing for DNA to be replicated. The researchers at first did not understand how two processes that should not be able to occur at the same time could be regulated by the same enzyme.
“What we uncovered was a really dynamic process where this enzyme was cycling on and off, allowing the cell to sometimes engage in metabolic activity and sometimes engage in DNA replication,” Cappell said.
The researchers determined that glycolysis is required to enter the cell cycle and begin replication, and that APC/C activity results in glycolysis inhibition. Using a biosensor, they tested the activity of APC/C and discovered that APC/C partially and transiently inactivates during the transition between G0 and G1, the first two phases of cell division. This transient inactivation allowed cells to coordinate both glycolysis and preparation for DNA replication before the transition into the third phase of the cell cycle, S phase. Furthermore, the biosensor showed that this transient inactivation only occurs in the first cell cycle after quiescence: if the cell divided more than once, this process did not recur, suggesting this mechanism is particularly important for cells just starting to proliferate.
This study is particularly relevant because glycolysis is thought to be used more extensively by cancer cells than normal cells, the authors said.
“We revealed a fundamental on-ramp mechanism for cell division: a precise, temporary flicker of the APC/C protein that enables a vital energy surge for the cell cycle to begin,” explained Paul. “Cancer cells often hijack this mechanism for their uncontrolled growth. By understanding this molecular switch, we can design therapies to specifically block cancer cells from initiating division.”
“This work also informs overcoming drug resistance,” he added. “If quiescent cancer cells use a version of this awakening switch to re-enter the cell cycle and divide faster, our findings offer new targets to prevent their reactivation, thereby combating relapse and improving treatment outcomes.”