News and Events
Proteins Released from the Nuclei of Dying Cancer Cells Promote Tumor Growth
Material released from dying cancer cells, known as tumor cell nuclear expulsion products (TuNEPs), contains specific proteins that promote the growth of neighboring cancer cells. Targeting these proteins could lead to new treatments that hinder cancer spread and improve patient outcomes.
Read MoreCelebrating CCR Careers: Len Neckers, Ph.D.
For over 40 years, Len Neckers, Ph.D., worked to develop molecularly targeted therapeutic approaches to modulate cancer cell growth and survival. He retired from CCR in November 2024.
Read MoreCCR researchers nominated to 2024 class of AAAS Fellows
Sheue-yann Cheng, Ph.D., Senior Investigator in the Laboratory of Molecular Biology, Anish Thomas, MBBS, M.D., Senior Investigator in the Developmental Therapeutics Branch, and Xin Wei Wang, Ph.D., Acting Co-Chief and Senior Investigator in the Laboratory of Human Carcinogenesis, were elected as 2024 Fellows to the American Association for the Advancement of Science (AAAS). Since 1874, the AAAS has elected scientists, engineers and innovators as Fellows for their achievements across many disciplines. Election as a Fellow honors members whose efforts on behalf of the advancement of science or its applications in service to society have distinguished them among their peers and colleagues.
Read MoreClinical trial researching drug therapy for neuroendocrine tumors
A clinical trial led by Frank I. Lin, M.D., Lasker Clinical Research Scholar in the Molecular Imaging Branch, is researching a drug therapy for adults with gastrointestinal neuroendocrine tumors and pheochromocytoma/paragangliomas.
Read MoreClinical trial researching immunotherapy for metastatic cancers
A clinical trial led by James L. Gulley., M.D., Ph.D., Co-Director and Senior Investigator in the Center for Immuno-Oncology, is researching immunotherapy for adults with locally advanced or metastatic cancers.
Read MoreElaine Jaffe and Ira Pastan elected as 2025 Fellows of the AACR Academy
Elaine S. Jaffe, M.D., NIH Distinguished Investigator in the Laboratory of Pathology, and Ira Pastan, M.D., Chief Emeritus of the Laboratory of Molecular Biology, were elected in the 2025 class of Fellows of the American Association for Cancer Research (AACR) Academy. The mission of the Academy is to recognize and honor extraordinary scientists whose groundbreaking contributions have driven significant innovation and progress in the fight against cancer. Jaffe is being recognized for her revolutionary research efforts in hematopathology and transforming diagnostic standards and care for lymphoma and leukemia; Pastan is being recognized for his visionary contributions to cancer therapy and the field of receptor biology.
Read MoreKumaran Ramamurthi elected to the American Academy of Microbiology
The American Academy of Microbiology elected Kumaran S. Ramamurthi, Ph.D., Deputy Chief and Senior Investigator in the Laboratory of Molecular Biology, as a 2025 fellow. Election to the Academy is a highly selective process based on records of scientific achievement and original contributions that have advanced the field of microbiology.
Read MoreCCR researchers receive Presidential Early Career Awards for Scientists and Engineers
Three CCR researchers received the Presidential Early Career Award for Scientists and Engineers (PECASE), one of the highest honors bestowed by the U.S. Government to outstanding scientists and engineers.
Read MoreNatasha Caplen appointed to Senior Investigator at CCR
The CCR community congratulates Natasha J. Caplen, Ph.D., who has been appointed Senior Investigator in the Genetics Branch. Caplen contributed to the discovery of RNA interference (RNAi) in mammalian cells and has pioneered approaches for exploiting this gene regulatory mechanism to investigate cancer biology and treatment. Her current studies focus on the functional genetic analysis of cancers driven by fusion oncogenes, particularly the pediatric tumor Ewing sarcoma.
Read MoreTargeting two proteins leaves some cancer cells vulnerable to chemotherapy
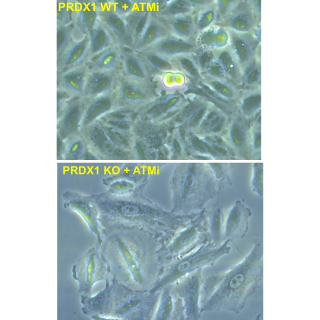
Targeting two proteins that cancer cells rely on to sustain their metabolism and DNA repair capabilities can double the survival rate of mice with lung cancer tumors. Cross referencing with data from The Cancer Genome Atlas of human tumors suggests that these findings could translate to people.
Read MoreResearch uncovers a novel mechanism for cells to recover from DNA damage
A newly discovered cellular mechanism sheds light on how cancer cells proliferate despite damage from anti-cancer treatments, providing a target for new therapies.
Read More