News and Events
New Strategy Shows Promise Against Deadly Breast Cancer in the Brain
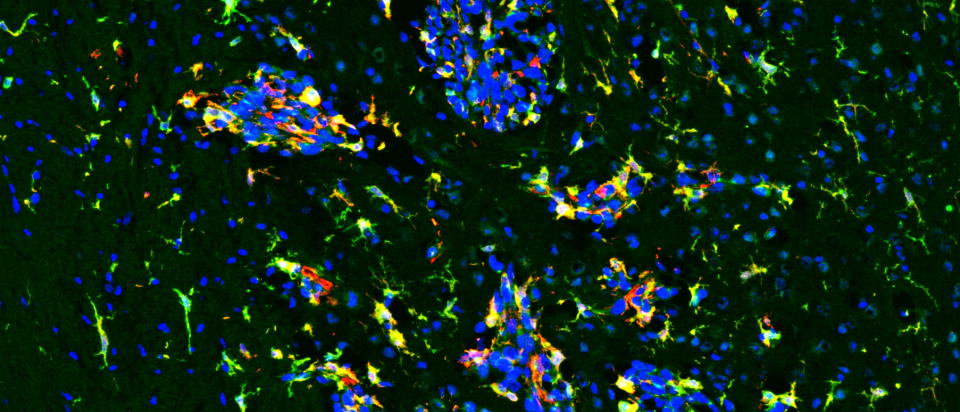
A new NIH study points to a promising strategy for treating aggressive breast cancer that spreads to the brain, a complication with few effective options. Learn how blocking a key brain cell survival pathway could open the door to future therapies.
Read MoreTargeting two proteins leaves some cancer cells vulnerable to chemotherapy
Targeting two proteins that cancer cells rely on to sustain their metabolism and DNA repair capabilities can double the survival rate of mice with lung cancer tumors. Cross referencing with data from The Cancer Genome Atlas of human tumors suggests that these findings could translate to people.
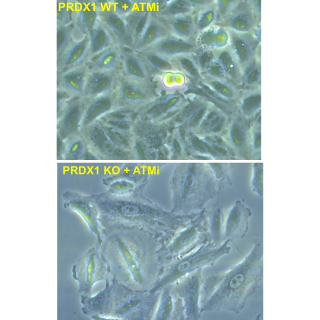
Read MoreResearch uncovers a novel mechanism for cells to recover from DNA damage
A newly discovered cellular mechanism sheds light on how cancer cells proliferate despite damage from anti-cancer treatments, providing a target for new therapies.
Read MoreCelebrating CCR Careers: Patricia S. Steeg, Ph.D.
Patricia S. Steeg, Ph.D., who discovered the first metastasis suppressor gene, nm23, announces her retirement.
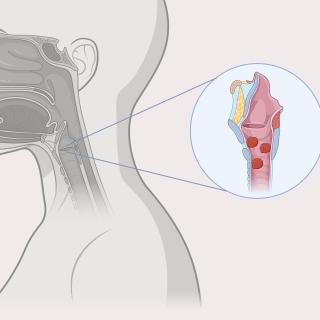
Read MoreInnovative gene therapy shows promise in treating patients with recurrent respiratory papillomatosis
A clinical trial led by CCR researchers demonstrated the effectiveness of the novel treatment, PRGN-2012, against recurrent respiratory papillomatosis in adults. The results serve as the foundation for an application for accelerated approval to the U.S. Food and Drug Administration (FDA).
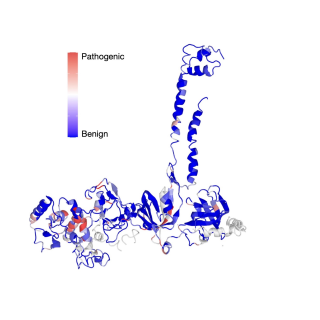
Read MoreResearchers categorize thousands of BRCA2 mutations as cancerous or benign
Researchers used CRISPR gene editing technology to determine whether over 6,000 variants of BRCA2 were likely to cause cancer.
Read MoreNirali Shah elected to the American Society for Clinical Investigation
Nirali N. Shah, M.D., M.H.Sc., Lasker Clinical Research Scholar in the Pediatric Oncology Branch, was elected as a member of the American Society for Clinical Investigation (ASCI). The organization seeks to support the scientific efforts, educational needs, and clinical aspirations of physician-scientists to improve the health of all people. The 2025 newly elected members came from 46 different institutions and represent excellence across the breadth of academic medicine.
Read MoreClinical trial researching immunotherapy for solid tumors
A clinical trial led by Danielle M. Pastor, D.O., Ph.D., Assistant Research Physician in the Center for Immuno-Oncology, is researching immunotherapy for adults with advanced solid tumors.
Read MoreClinical trial researching combination drug therapy for neuroendocrine carcinomas
A clinical trial led by Jaydira Del Rivero, M.D., Associate Research Physician in the Developmental Therapeutics Branch, is researching combination drug therapy for adults with high-grade neuroendocrine carcinomas.
Read MoreDrug shrinks nerve tumors in adults with neurofibromatosis type 1 in a clinical trial
Until recently, no effective treatments existed for non-cancerous inoperable nerve tumors, called plexiform neurofibroma, in adults. A clinical trial of the drug selumetinib shows it can cause tumor shrinkage and relieve symptoms, such as pain.
Read MoreCelebrating CCR Careers: Jay A. Berzofsky, M.D., Ph.D.
Jay A. Berzofsky, M.D., Ph.D., a chemist-turned-immunologist who pioneered cancer immunology and immunotherapy strategies, announces his retirement from the NCI.
Read More