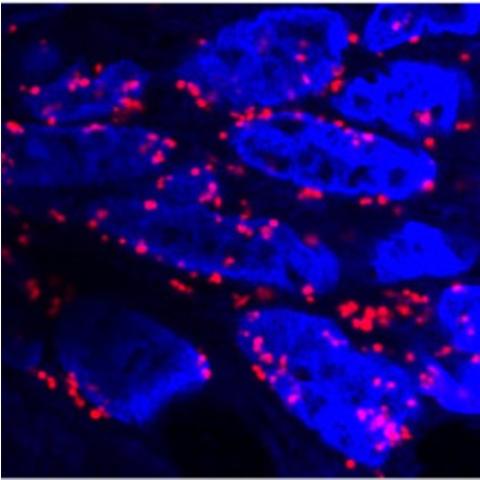
CCR researchers showed that RAS can colocalize with RAN-GAP (red dots) at the edge of the cell nucleus (bright blue), where it helps trigger nuclear transport machinery to release cargo proteins such as EZH2 into the cytoplasm. Image credit: Tripathi et al.
A team of researchers led by Doug Lowy, M.D., Chief of the Laboratory of Cellular Oncology and Principal Deputy Director of the National Cancer Institute, has discovered a surprising way that one of the best studied oncoproteins in human cancer, RAS, can support the growth of cancer cells. Aberrant RAS proteins, found in 20% to 30% of cancers, are best known for relaying cancer-promoting signals from cells’ outer membranes. In a study published online November 11, 2024, in Nature Cancer, Lowy, staff scientist Brajendra Tripathi, Ph.D., and their colleagues report that mutated RAS can also promote the export of proteins from a cell’s nucleus to its cytoplasm, where some can contribute to cancerous growth.
In the lung cancer cells that the team studied, one consequence of this molecular relocation was the degradation of a tumor-suppressing protein called DLC1. Lowy says it may be possible to exploit this previously unrecognized role of RAS to develop combination therapies that boost the anti-cancer effects of RAS-inhibiting drugs.
Two FDA-approved cancer therapies are currently available to rein in RAS signaling, but most patients’ cancers eventually become resistant to these drugs. “The next step is finding favorable combinations of treatments: ‘RAS-inhibitor-plus,’ if you will,” Lowy says.
The discovery of RAS’s role in nuclear export began with studies of the DLC1 tumor suppressor. Experiments in Lowy’s lab showed that an enzyme called EZH2 was acting on DLC1 in the cytoplasm of lung cancer cells — a surprising finding, because EZH2 is often considered to be only a nuclear protein.
When the team investigated how EZH2 was getting out of the nucleus and into the cytoplasm of the cancer cells, their experiments implicated RAS. They found that the RAS protein helps trigger nuclear transport machinery to release cargo proteins such as EZH2 into the cytoplasm after they are ferried across the membrane that surrounds the cell’s nucleus.
The displacement of EZH2 into the cytoplasm is important because EZH2’s interaction with DLC1 makes the tumor suppressor vulnerable to destruction. By keeping EZH2 out of the cytoplasm, RAS inhibitors could stop the degradation of DLC1. But based on earlier work, the researchers knew that would not be enough to restore its tumor suppressor function.
To keep DLC1 working to fend off cancerous growth, a dual approach was necessary. For this, the team turned to drugs that target two other DLC1-regulating proteins, SRC and AKT. The team found that delivering SRC and AKT inhibitors along with RAS inhibitors slowed the growth of lung cancer cells more effectively than RAS inhibitors alone, both in lab-grown cells and in mice.
Lowy suspects RAS might skew the cellular distribution of proteins in many kinds of cancer. Supporting this idea, his team and collaborators at Emory University in Atlanta have found RAS bound to a partner protein needed to facilitate its role in nuclear export in several different tumor models and patient samples. Now, he says, a priority will be to explore whether combining RAS inhibitors with DLC1-activating drugs might also be effective against laboratory models of pancreatic cancer, a disease for which new treatments are much needed.