
Brajendra Tripathi, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 37, Room 4112
- Bethesda, MD 20892
- 240-760-7931
- 240-541-4502
- tripathib@mail.nih.gov
RESEARCH SUMMARY
Dr. Tripathi has an abiding interest in the clinical applications of basic research. His current research is mainly focused on how tumor suppressor proteins, which are downregulated or inactivated in several cancer types by distinct mechanisms, can be stabilized and reactivated and exploited for cancer treatment. Specifically, Dr. Tripathi and colleagues are exploring functions of the tumor suppressor protein Deleted in Liver Cancer 1 (DLC1) and the kinases and methyltransferases that regulate its functions in normal physiology and in cancer.
Areas of Expertise
Biography

Brajendra Tripathi, Ph.D.
After earning his M.Sc. in Biochemistry, Dr. Tripathi joined the graduate program in Biochemistry and Molecular & Cell Biology at the CSIR-Central Drug Research Institute, India, where he was a recipient of the Council of Scientific and Industrial Research Fellowship.
Dr. Tripathi’s graduate research work elucidated molecular and biochemical mechanisms of insulin sensitivity and insulin resistance in alcoholism, which resulted in several peer-reviewed publications, and he received his Ph.D. in Biochemistry from the prestigious Jawaharlal Nehru University, New Delhi, India. Dr. Tripathi carried out his postdoctoral research in the Laboratory of Molecular and Developmental Biology at the National Eye Institute, National Institutes of Health, where his research was focused on signal transduction mechanisms that regulate epithelial cell adhesion and migration in the lens and cornea. He has developed animal models for studying corneal wound healing and evaluated the therapeutic potential of inhibitors of protein kinases, which regulate cell adhesion and cell migration. Subsequently, Dr. Tripathi joined the Laboratory of Cellular Oncology at the National Cancer Institute as a Research Fellow (FTE) and was promoted to Staff Scientist.
Dr. Tripathi has authored over 35 peer-reviewed research articles and is a co-inventor in 14 patents. Dr. Tripathi is a recipient of numerous awards, including the NCI Director's Innovation Award, two NCI Federal Technology Transfer Awards, four Distinguished Achievement Awards, the Fellow Award for Research Excellence, the American Society of Cell Biology Travel Award, and the National Eye Institute Scientific Director’s Outstanding Mentor Award and has been featured in the ‘People and Ideas’ section of the Journal of Cell Biology. Dr. Tripathi has supervised and mentored many postbaccalaureate fellows and postgraduate research trainees. Dr. Tripathi is currently serving as an Associate Editor and Editorial Board member of several scientific journals and is an active member of the Staff Scientist and Staff Clinician (SSSC) community at the Center for Cancer Research, National Cancer Institute.
News
Dr. Tripathi is featured in the ‘People and Ideas’ section of the Journal of Cell Biology.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6781442/
Dr. Tripathi and colleagues research is featured in the special issue “Cancer Cell Biology 2020” of the Journal of Cell Biology: SRC targets the tumor suppressor DLC1: Interaction between DLC1 and tumor promoting kinase SRC reveals a possible new treatment route for DLC1-positive tumors.
Covers
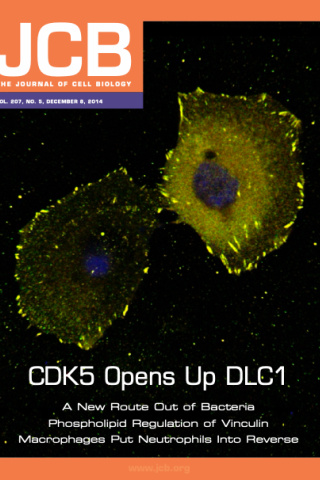
CDK5 Opens Up DLC1
Tripathi et al. reveal that the serine/threonine kinase CDK5 phosphorylates the tumor suppressor DLC1 (red), switching it from a closed to an open conformation and thereby promoting its Rho-GAP activity, its ability to bind tensin and talin, and its localization to focal adhesions (green). Colocalization (yellow) of DLC1 protein with the focal adhesion protein Vinculin. Nuclei are labeled blue.
Brajendra K. Tripathi*, Xiaolan Qian, Philipp Mertins, Dunrui Wang, Alex G. Papageorge, Steven Carr, and Douglas R. Lowy* (2014) Journal of Cell Biology. 207(5): 627-642. doi: 10.1083/jcb.201405105
Research
The DLC1 tumor suppressor protein has a critical role in growth regulation and metastasis, and the attenuation of its tumor suppressor activities by several oncogenic kinases, such as CDK5, SRC, ERK, and AKT, strongly suggests that DLC1 reactivation by inhibitors of these kinases could be useful for cancer treatment. Dr. Tripathi’s research made many fundamental discoveries and revealed the critical role that some protein kinases and methyltransferases play in both normal development and cancer. Specifically, his research projects elucidate the molecular mechanisms by which DLC1 tumor suppressor protein is inactivated or degraded in lung cancer, and he designs hypothesis-based experimental approaches to study lung cancer treatment. Mutant KRAS is found in more than 30% of lung adenocarcinomas, and tumors with mutant KRAS usually have a poor prognosis with current therapies. In many solid tumors such as mutant KRAS lung adenocarcinoma, expression of DLC1 tumor suppressor protein is downregulated post-translationally by distinct mechanisms including ubiquitin-dependent proteasomal degradation. A major goal of Dr. Tripathi’s research is the preclinical testing of relevant combinations of inhibitors that can stabilized and reactivate the DLC1 tumor suppressor protein in cancer models that harbor mutant KRAS. His research intends to investigate the molecular signaling impacted by the combination of inhibitors and to determine the degree to which the antitumor activity of the inhibitors depends on DLC1 protein. For these studies, Dr. Tripathi has developed several cell-based assays, isogenic gene knockout lines, and animal models, and is collaborating with several established investigators working at the NIH intramural and extramural laboratories.
