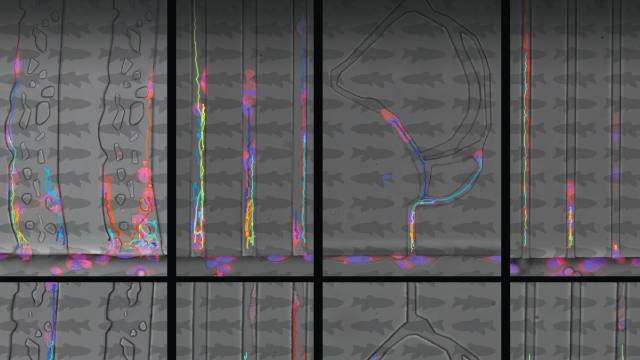
Where and how fast a protein is made in the cell affects what it does.

Messenger RNA (mRNA, yellow dots) in the two breast cancer cells depicted here holds the instructions to make NET1 protein. New research has demonstrated that the location in a cell where this mRNA is turned into protein and the speed at which that process occurs affects what job the protein will have. By altering the location and speed of protein production, researchers may be able to control the protein’s function and believe this could be harnessed for therapeutic purposes. Credit: Alexander N. Gasparski, CCR, NCI, NIH; SPGM, FNL, NCI, NIH
It has been generally assumed that where in the cell proteins are made does not matter much for their function, especially because proteins can move quite freely from one place to another. But new research by Senior Investigator Stavroula Mili, Ph.D., and her colleagues now shows that the location and even the speed of how a protein is made in a cell influences what the protein ends up doing.
“If we understand more about the mechanisms that cells use to determine protein function, we could maybe manipulate these mechanisms and channel proteins to specific functions for therapeutic purposes,” Mili says.
The instructions on how to construct proteins in the cell are encoded in messenger RNA (mRNA), which is distributed throughout a cell. Mili and her team were studying the mRNA that encodes a protein called NET1, which is associated with the spread of cancer cells, when they noticed that it can be found either at the center of the cell near the nucleus, or further away, in the cell periphery near the cell membrane. They were curious to understand if the location of the mRNA influenced the resulting protein's function.
Indeed, the researchers found the NET1 mRNA near the cell nucleus produced NET1 protein that interacted more with a protein known to help bring other proteins into the nucleus, called importin beta1. NET1 synthesized in this location was thus transported and sequestered into the nucleus, where it is thought to control cellular responses to DNA damage.
On the other hand, when NET1 mRNA was located further away from the nucleus, it gave rise to NET1 protein that interacted more with a different protein partner called CASK, which prevented its interaction with importin beta1 and transport into the nucleus. In this latter case, NET1’s function could augment the cancer cells’ ability to move, which is a property important for cancer metastasis.
But it is not only the location of where a protein is made that matters — the speed of protein production also seems to have consequences. Mili and colleagues manipulated the rate at which NET1 mRNA is translated into NET1 protein. Strikingly, slow synthesis — regardless of mRNA location — resulted in NET1 protein that interacted with importin beta1 and was carried into the cell nucleus, while relatively faster synthesis resulted in the opposite effect, decreasing importin beta1 binding and nuclear targeting.
Together, these experiments, described in a study in Molecular Cell, suggest that both the location and rate of protein synthesis can affect which proteins interact with each other, which in turn influences a protein’s function.
Moving forward, Mili plans to explore this phenomenon in greater detail. “We are trying to understand how widely applicable these mechanisms are — do they play a role in regulating other protein functions? We’re also studying these mechanisms to see if we can use them to affect the ability of cancer cells to metastasize,” she says.