A new view of cell division changes the way we think about how some cancer drugs impact cells.

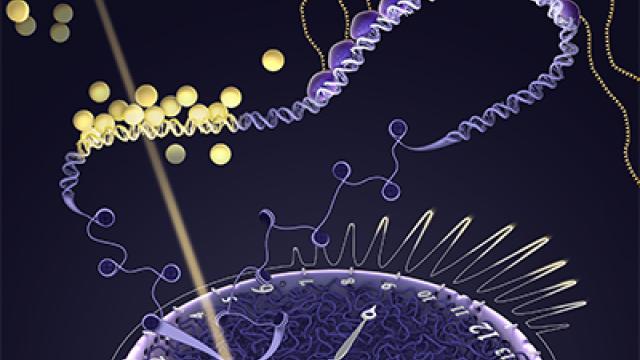
Past a certain point in a cell’s cycle of growth and division, it had been assumed that the cycle cannot be stopped and that a cell must undergo mitosis, or cell division, due to continuous CDK2 signaling. New research, however, shows that inhibiting the growth signaling proteins CDK4 and CDK6 can reverse the cell’s decision to divide — if the signals are stopped early in the cell cycle. These two stopwatches show samples of cell activity over time, with yellow indicating progress toward mitosis and blue indicating a resting stage. In the group of cells treated with a CDK4/6 inhibiting drug (right), up to 15% of cells gradually lost CDK2 activity, returned to a resting state and did not enter mitosis. This did not occur when cells in the group to the left were untreated. Credit: James A. Cornwell, CCR, NCI, NIH; SPGM, FNL, NCI, NIH
Looking at familiar processes in new ways sometimes overturns long-standing ideas about how biology works. That’s what happened when Stadtman Tenure-Track Investigator Steve Cappell, Ph.D., followed individual cells in real time as they proceeded through the cycle of cell growth and division. His team unexpectedly discovered that cells that are preparing to divide are not as committed to completing that process as previously thought. Instead, they reported in Nature, a cell’s decision to divide can be reversed.
When cells receive growth-promoting signals, they begin to prepare for cell division by entering the cell cycle. That cycle is a precisely choreographed series of steps that culminates in a step called mitosis, which divides a cell in two. The textbook view of the cell cycle includes a point of no return after which growth signals would no longer be needed to drive cells to divide. But James Cornwell, Ph.D., a postdoctoral researcher in Cappell’s lab, noticed that not all cells follow this rule. When he took growth signals away from growing cells, some of the cells managed to exit the cell cycle and return to a resting state, even when they had progressed beyond the supposed point of no return.
Cornwell took advantage of the advanced imaging technology available in CCR to capture videos of thousands of cells and monitor each one’s progression individually through the cell cycle. When cells stopped receiving growth signals, most continued through the cell cycle and eventually split, but cells that were still early in the cycle when they stopped receiving growth signals returned to a resting state.
The team discovered that each cell’s fate is essentially a race between the steps leading up to mitosis and the steps necessary for cell cycle exit. “What we found is that usually mitosis wins that race, but sometimes the exit will win,” Cappell explains. Cells that had just started to prepare for mitosis had the best chance of completing the exit process before time ran out. But when the researchers stopped the clock by blocking mitosis entirely, even cells that were farther along when growth signals were interrupted managed to exit the cycle.
In addition to overturning a long-held dogma of how cells grow and divide, the team’s discovery could lead to better cancer therapies. The researchers identified specific molecules that control the race between mitosis and cell cycle exit, and their findings suggest that drugs that work by inhibiting certain cell cycle regulators — CDK4 and CDK6 — likely affect cells’ progression through the cell cycle differently than previously thought. “It turns out, we don't quite understand all the pathways that cells can take when we treat them with these drugs,” Cappell says. Recognizing how such drugs might affect the fates of individual cancer cells could allow Cappell and his team to design combination therapies with longer-lasting benefits for patients.