Clinical Trials
Clinical trial researches drug therapy for bone marrow neoplasms
A clinical trial led by Steven Z. Pavletic, M.D., Senior Clinician in the Immune Deficiency Cellular Therapy Program, is researching a therapy for myelodysplastic syndromes (MDS), a group of bone marrow neoplasms.
Read MoreClinical trial researching therapy for prostate cancer
A clinical trial led by Ravi A. Madan, M.D., Senior Clinician in the Genitourinary Malignancies Branch, is researching a combination drug therapy for prostate cancer.
Read MoreClinical trial researching therapy for cancers of the reproductive system
A clinical trial led by Jung-Min Lee, M.D., Lasker Clinical Research Scholar in the Women’s Malignancies Branch, is researching a combination drug therapy for cancers of the ovaries, fallopian tubes or peritoneum.
Read MoreClinical trial researches immunotherapy for recurrent thymoma and thymic carcinoma
A clinical trial led by Arun Rajan, M.D., Senior Clinician in the Thoracic and GI Malignancies Branch, is researching treatments, including immunotherapy, for recurrent thymoma and thymic carcinoma.
Read MoreClinical trial researching immunotherapy for B-cell lymphoma
A clinical trial led by Mark Roschewski, M.D., Senior Clinician in the Lymphoid Malignancies Branch, is researching immunotherapy for indolent B-cell lymphoma, a cancer of white blood cells that frequently relapses.
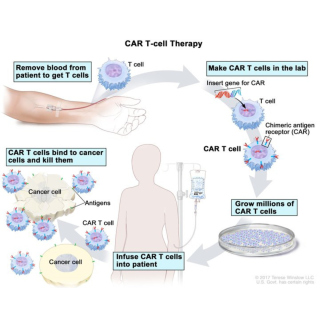
Read MoreClinical trial researches CAR T-cell therapy for hairy cell leukemia
Robert J. Kreitman, M.D., Senior Investigator in the Laboratory of Molecular Biology, is leading a study to see whether CAR T-cell therapy impacts patients with hairy cell leukemia.
Read MoreClinical trials research immunotherapy for Kaposi sarcoma
Clinical trials led by Ramya Ramaswami, M.B.B.S., M.P.H., Physician-Scientist Early Investigator in the HIV and AIDS Malignancy Branch, are researching immunotherapy for Kaposi sarcoma.
Read MoreClinical trial researching PSMA imaging in prostate cancer
A clinical trial led by Deborah E. Citrin, M.D., Senior Investigator in the Radiation Oncology Branch, is researching prostate-specific membrane antigen (PSMA) imaging to evaluate response to radiation treatment in patients with prostate cancer.
Read MoreClinical trial researching therapy for some small cell lung cancers
Anish Thomas, M.B.B.S., M.D., Lasker Clinical Research Scholar in the Developmental Therapeutics Branch, is leading NCI’s participation in a trial researching therapies for small cell lung cancer.
Read MoreClinical trial researches therapy for VEXAS syndrome
A clinical trial led by Dennis Hickstein, M.D., Senior Investigator in the Immune Deficiency Cellular Therapy Program, is researching a therapy for people with VEXAS (Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic) syndrome.
Read More