Image credit: iStock
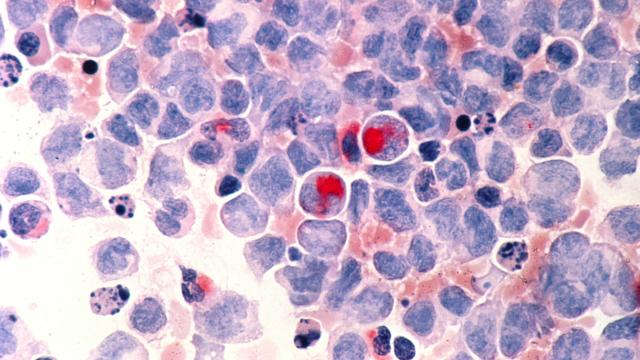
VEXAS (Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic) syndrome is an adult-onset disease characterized by a number of inflammatory and hematologic symptoms. A clinical trial led by Dennis Hickstein, M.D., Senior Investigator in the Immune Deficiency Cellular Therapy Program, is researching stem cell therapy for patients with this disease. The trial will take place at the NIH Clinical Center in Bethesda, Maryland, and there is no cost for participation.
For more information, please contact Dr. Hickstein at (240) 760-6169 or hicksted@mail.nih.gov
Clinicaltrials.gov identifier: NCT05027945
NCI Protocol ID: NCI000404
Official Title: A Phase II Study of Allogeneic Hematopoietic Stem Cell Transplant for Subjects With VEXAS (Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic) Syndrome
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.