Stem cell transplant
Image source: NCI Visuals Online
Patients with a known deleterious genetic mutation in a gene identified in a primary immunodeficiency disease, or who have the phenotype of a primary immunodeficiency disease with life-threatening opportunistic infections or a viral-derived cancer, are eligible to participate in this clinical trial at the NIH Clinical Center.
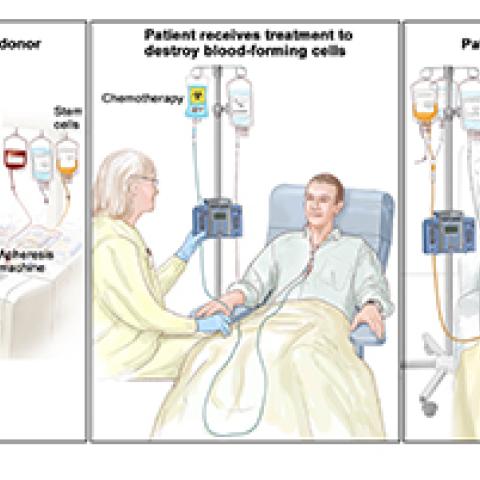
Primary immunodeficiency diseases (PIDs) are rare genetic disorders that impair the immune system. Dennis D. Hickstein, M.D., Senior Investigator in the Immune Deficiency Cellular Therapy Program is leading a study that uses new DNA technology that speeds up the process of screening for PIDs and finding an acceptable donor match for hematopoietic stem cell transplant (HSCT). The goal is to see if HSCT that uses donor cells is safe and can rebuild normal immune function in patients with PID. People who don’t have a functioning immune response can be subject to chronic, debilitating infections that can increase the risk of cancer. PIDs are hematologic diseases—disorders of the blood and the organs that form blood, such as bone marrow. HSCT is a recognized treatment for PIDs. Allogeneic HSCT uses blood or bone marrow from a healthy donor, usually a relative of the patient. To prevent the patient’s body from rejecting these “foreign” cells, the patient is first treated with high doses of chemotherapy, radiation and other drugs that destroy diseased cells and suppress any remaining immune response that could attack the donated cells. The patient then receives an infusion of new stem cells from a healthy donor to rebuild healthy blood and normal immunity.
Clinicaltrials.gov identifier: NCT04339777
NCI Protocol ID: NCI-20-C-0070
Official Title: A Phase II Study of Allogeneic Hematopoietic Stem Cell Transplant for Patients With Primary Immunodeficiency Diseases
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.