Clinical Trials
Clinical trial researching therapy for aggressive gliomas
A clinical trial led by Sadhana Jackson, M.D., Adjunct Investigator in the Pediatric Oncology Branch, is researching a drug therapy for diffuse midline gliomas, a type of aggressive brain tumor.
Read MoreClinical trial researching therapy for HIV-associated blood cancers
A clinical trial led by Kathryn A. Lurain, M.D., M.P.H., Assistant Research Physician in the HIV and AIDS Malignancies Branch, is researching a combination drug therapy for HIV-associated B-cell non-Hodgkin lymphomas.
Read MoreClinical trial researches combination drug therapy for B-cell lymphoma of the central nervous system
A clinical trial led by Mark Roschewski, M.D., Senior Clinician in the Lymphoid Malignancies Branch, is researching a therapy for B-cell lymphoma of the central nervous system.
Read MoreFirst clinical trial testing a prevention for breast cancer metastasis to the brain yields encouraging results
When breast cancer metastasizes to the brain, new tumors usually develop even after treatment. But recurrence was low among women who received low-dose temozolomide with T-DM1 in a phase I clinical trial.
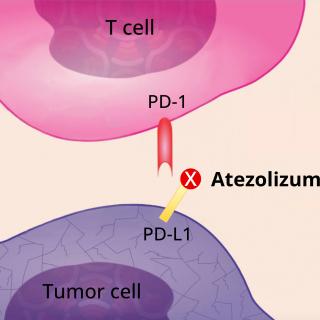
Read MoreNCI clinical trial leads to atezolizumab approval for advanced alveolar soft part sarcoma
NCI and CCR researchers have played an instrumental role in identifying the first treatment approved for advanced alveolar soft part sarcoma. The immunotherapy drug atezolizumab (Tecentriq) was approved by the U.S. Food and Drug Administration for the treatment of adults and children two years of age and older with alveolar soft part sarcoma that has spread to other parts of the body or cannot be removed by surgery. The CCR Pediatric Oncology Branch provided treatment and care for all trial patients under 18 years old, making this the first approval for atezolizumab in children.
Read MoreClinical trial researching combination drug therapy for multiple myeloma
A clinical trial led by Elizabeth M. Hill, M.D., Assistant Research Physician in the Lymphoid Malignancies Branch, is researching hematopoietic stem cell transplants and therapy for acute myeloid leukemia.
Read MoreClinical trial researching therapy for bile duct cancer
A clinical trial led by Jonathan Hernandez, M.D., Investigator in the Surgical Oncology Program, is researching a combination of immunotherapy and chemotherapy for liver-only cholangiocarcinoma.
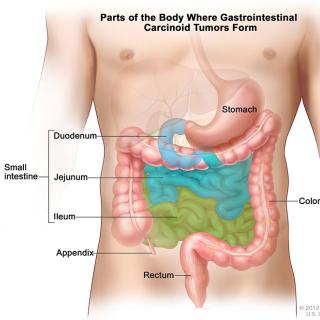
Read MoreClinical trial evaluates drug combination for inoperable neuroendocrine tumors
Neuroendocrine tumors occur in cells of the endocrine and nervous systems. A combined phase I/II clinical trial at the NIH Clinical Center evaluates the effectiveness of a combination of two agents that may work in complementary ways to target inoperable or metastatic neuroendocrine tumors.
Read MoreClinical trial researching T-cell therapy for sarcoma
John W. Glod, M.D., Ph.D., Associate Research Physician in the Pediatric Oncology Branch, is leading NCI’s participation in a clinical trial researching T-cell therapy for sarcomas.
Read MoreClinical trial researching therapy for tumors that have spread to the lining of the abdomen
A clinical trial led by Andrew M. Blakely, M.D., Assistant Research Physician in the Surgical Oncology Program, is researching a combination drug therapy for peritoneal carcinomatosis, a condition in which tumors have spread along the lining of the abdomen from the appendix, colon, ovary or mesothelioma.
Read More