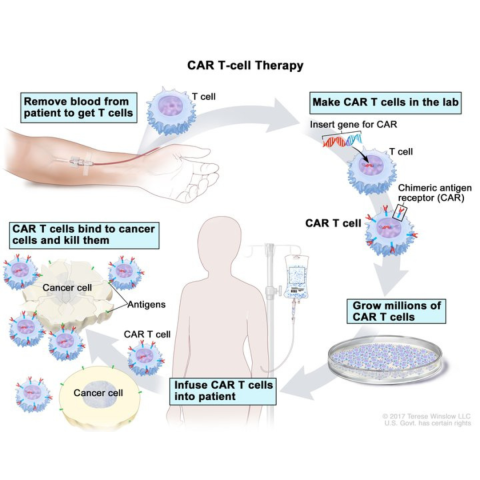
Drawing of blood being removed from a vein in a patient’s arm to get T cells. Also shown is a special receptor called a chimeric antigen receptor (CAR) being made in the laboratory; the gene for CAR is inserted into the T cells and then millions of CAR T cells are grown. Drawing also shows the CAR T cells being given to the patient by infusion and binding to antigens on the cancer cells and killing them.
Photo credit: NCI Visuals Online, Terese Winslow
Hairy cell leukemia (HCL) is a slow-growing cancer of the blood in which the bone marrow makes too many B cells, a type of white blood cell that fights infection. These excess B cells look abnormally “hairy” under a microscope. High-risk HCL and more aggressive variants respond poorly to chemotherapy, and people with high-risk HCL tend to have a poor prognosis. Robert J. Kreitman, M.D., Senior Investigator in the Laboratory of Molecular Biology, is leading a study to see whether CAR T-cell therapy impacts participants whose HCL has returned after treatment or no longer responds to standard treatments.
T cells are part of the immune system and are responsible for finding and destroying abnormal cells, including cancer cells. However, sometimes cancer cells find ways to hide from the immune system. CAR T-cell therapy is a way to program the immune system to attack cancer cells. T cells are collected from the blood of a patient with HCL and are genetically engineered to sprout chimeric antigen receptors (CARs) on their surfaces. Then these modified T cells are reinjected into the patient. Investigators want to find out if the CARs can help T cells do a better job of identifying and attacking HCL cells throughout the body and if giving escalating doses of CAR T cells is safe.
Recent interim results were published in July 2025: Complete Response in Hairy Cell Leukemia to Anti-CD22 CAR T-Cell Therapy (Kreitman RJ, et al. JCO Precis Oncol. 2025 Jul;9:e2500269. Epub 2025 Jul 16.)
Clinicaltrials.gov identifier: NCT04815356
NCI Protocol ID: NCI-21-C-0019
Official Title: Phase I Study of Anti-CD22 Chimeric Receptor T Cells in Patients With Relapsed/Refractory Hairy Cell Leukemia and Variant
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.