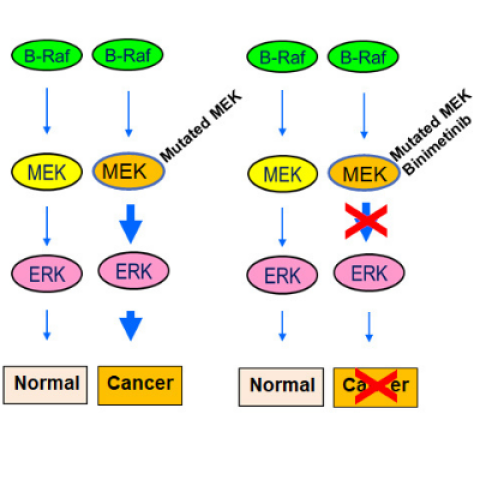
Normally BRAF (green) stimulates (phosphorylates) MEK (yellow), and in turn, MEK stimulates ERK (pink), leading to normal cell proliferation. An overactive MEK, possibly due to a mutation, overstimulates ERK, leading to increased cell proliferation. This may cause some forms of hairy cell leukemia (HCL) or its variants (HCLv). Inhibition of MEK by Binimetinib blocks the overstimulation of ERK. This leads to the death of the HCL/HCLv cells.
Image credit: Robert Kreitman, M.D.
Adults with hairy cell leukemia without a BRAF gene mutation and whose disease has returned or not responded to treatment may be eligible to participate in a clinical trial at the NIH Clinical Center.
Robert J. Kreitman, M.D., Senior Investigator in the Laboratory of Molecular Biology, is leading a study of a treatment for hairy cell leukemia (HCL). HCL is a rare, slow-growing cancer of the blood in which a person’s bone marrow makes too many B cells, a type of white blood cell that fights infection. These extra B cells are abnormal and look “hairy” under a microscope. Most people with HCL have a BRAF gene mutation. There are drugs that target this mutation, but they do not help people with HCL who do not have a BRAF gene mutation. However, when the BRAF gene is not mutated in people with HCL, frequently the MEK gene is. Binimetinib is a MEK inhibitor that interferes with signals the MEK gene sends that help to drive the growth of cancer cells. Investigators want to see if interfering with MEK signaling can kill abnormal “hairy” B cells. The goal of this study is to determine the overall rate of response to binimetinib in patients with HCL who do not have a BRAF gene mutation.
Clinicaltrials.gov identifier: NCT04322383
NCI Protocol ID: NCI-20-C-0075
Official Title: Phase 2 Trial for Binimetinib for Patients With Relapsed/Refractory BRAF Wild Type Hairy Cell Leukemia and Variant
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.