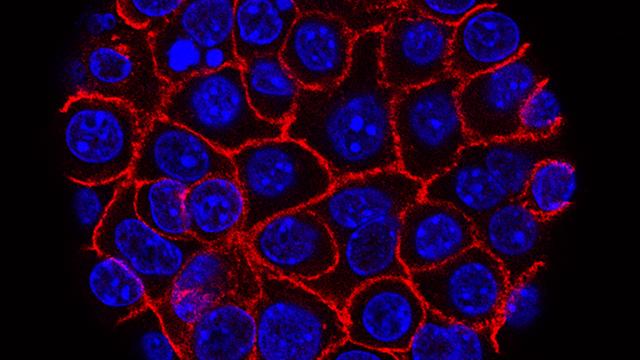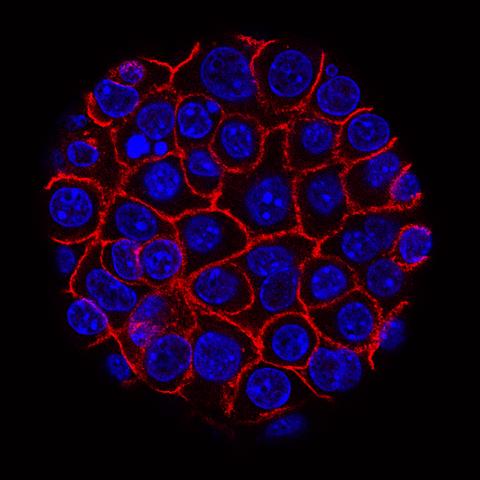
This image shows pancreatic cancer cells (nuclei in blue) growing as a sphere encased in membranes (red). By growing cancer cells in the lab, researchers can study factors that promote and prevent the formation of deadly tumors.
Image Source: NCI Visuals Online
Adults with some solid tumors may be eligible to participate in a clinical trial at the NIH Clinical Center.
Christine Alewine, M.D., Ph.D., a Lasker Clinical Research Scholar in the Laboratory of Molecular Biology, is leading the CCR participation in a trial to evaluate a drug for therapy of pancreatic cancer and other solid tumors. Some tumors release signals that increase the amount of blood vessels in the tumor. Blood vessels are like highways that deliver nutrients, so increasing their number gives cancer cells more fuel to grow. Other tumors use nearby cells called fibroblasts to create a thick physical barrier that helps to hide the tumor from the body’s immune system, and it’s so dense that most drugs can’t penetrate it to attack the cancer. ProAgio, the study drug, works by targeting αvβ3 (alpha-v beta-3) integrin, which is present on the surface of the abnormal endothelial cells that form tumor blood vessels and also on cancer-associated fibroblasts that generate the physical barrier. ProAgio causes these cancer-supporting cells to die. This inhibits the growth of tumors and allows other drugs to be more effective. Researchers want to see if ProAgio is safe and to find the most effective dose.
Clinicaltrials.gov identifier: NCT05085548
NCI Protocol ID: NCI000194
Official Title: A Phase I Trial of ProAgio, an Anti-αvβ3 Integrin Cytotoxin, for Previously Treated Advanced Pancreatic Cancer and Other Solid Tumor Malignancies
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.