Clinical Trials
Clinical trial researches three-part immunotherapy regimen for gastric or head and neck cancer
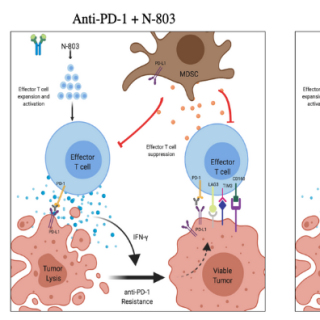
Jason M. Redman, M.D., Assistant Research Physician in the Genitourinary Malignancies Branch, is leading a study researching a three-part immunotherapy regimen in adults with gastric or head and neck cancer.
Read MoreClinical trial aims to reduce hormone therapy side effects for people with prostate cancer
A team at CCR is investigating a new device to deliver hormone therapy directly into prostate cancer tumors. The approach could improve patient quality of life by reducing burdensome side effects of current hormone therapies.
Read MoreFirst-in-human clinical trial evaluates drug for pancreatic cancer and other solid tumors
Christine Alewine, M.D., Ph.D., a Lasker Clinical Research Scholar in the Laboratory of Molecular Biology, is leading CCR participation in a first-in-human trial to evaluate a drug for therapy of pancreatic cancer and other solid tumors. The drug aims to attack a physical barrier that helps to hide the tumor from the body’s immune system.
Read MoreSteven Pavletic named Top Ten Clinical Research Achievement awardee
Steven Z. Pavletic, M.D., M.S., Senior Clinician in the Immune Deficiency Cellular Therapy Program, has been named a Top Ten Clinical Research Achievement awardee by the Clinical Research Forum for his study “A randomized phase 2 trial of pomalidomide in subjects failing prior therapy for chronic graft-versus-host disease.”
Read MoreClinical trial studies combination therapy for relapsed/refractory B-cell lymphomas
Burkitt lymphoma and diffuse large B-cell lymphoma are fast-growing cancers, and new therapies are needed for adults whose disease is not effectively treated with chemotherapy alone. Investigators at CCR are studying a combination therapy as a potential treatment for people with these aggressive cancers.
Read MoreLiving with and learning about neuroendocrine tumors: A conversation with Dr. Jaydira Del Rivero
Jaydira Del Rivero, M.D., Assistant Research Physician in the Developmental Therapeutics Branch, studies neuroendocrine tumors – a rare group of malignant neoplasms that originates from neuroendocrine cells and affects almost any part of the body. In this Q&A, Dr. Del Rivero and a natural history trial participant discuss an ongoing neuroendocrine study and their experiences at NIH.
Read MoreClinical trial evaluates drug for treatment of neurofibromatosis type 1 and atypical neurofibromas
Up to half of the people with neurofibromatosis type 1 develop plexiform neurofibromas (PNs), tumors that grow along nerves. Over time, PNs may transform into atypical neurofibromas (ANFs) and then into cancerous tumors. Andrea Gross, M.D., Assistant Research Physician in the Pediatric Oncology Branch, is leading a study of a drug, abemaciclib, to treat ANFs in children.
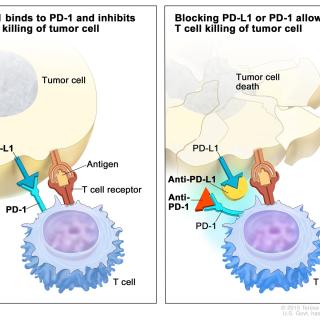
Read MoreClinical trial compares interval dosing of two immune checkpoint inhibitors
William D. Figg Sr., Pharm.D., Deputy Chief of the Genitourinary Malignancies Branch, is leading CCR’s efforts in a multi-institution, international study comparing methods of dosing nivolumab and pembrolizumab. This study is intended for people who have advanced or metastatic cancer that has not been previously treated with an immune checkpoint inhibitor.
Read MoreNCI study advances personalized immunotherapy for metastatic breast cancer
An experimental form of immunotherapy that uses an individual’s own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer, according to results from an ongoing clinical trial led by Steven A. Rosenberg, M.D., Ph.D., Chief of the Surgery Branch.
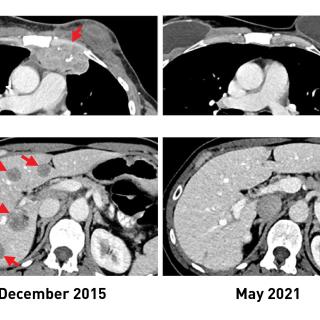
Read MoreAll about the drive: A rare kidney cancer meets its match at NIH
On New Year’s Eve, 2020, Katie Coleman was diagnosed with a very rare form of advanced kidney cancer. She enrolled in a clinical trial led by a collaborative CCR team that gave her the opportunity to have surgery in hopes of removing her tumors.
Read More