Computer illustration of lung cancer.
Photo credit: Science photo library
Adults with advanced solid tumors may be eligible to participate in a clinical trial at the NIH Clinical Center.
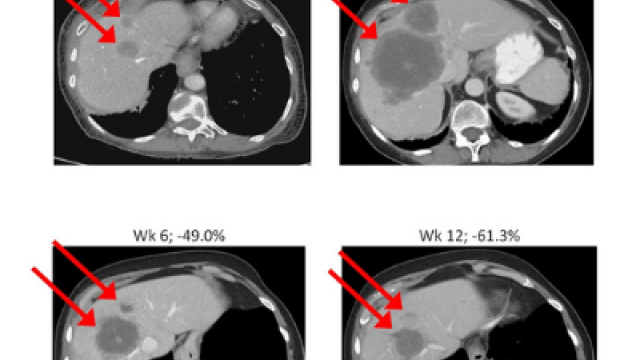
Defects in DNA repair pathways enable cancer cells to accumulate genomic alterations. Several cancer treatments, including chemotherapy and radiation, take advantage of cancer’s defective DNA damage repair. More recently, treatment of these cancers has improved due to the discovery of drugs called PARP inhibitors, which block a back-up DNA repair mechanism. However, in many cases, the cancer eventually develops resistance to this treatment and the tumor starts to regrow. Anish Thomas, M.B.B.S., M.D., Lasker Clinical Research Scholar in the Developmental Therapeutics Branch, is leading a study using berzosertib in combination with sacituzumab govitecan to target ATR, a key protein involved in an alternative DNA repair pathway used by chemotherapy and PARP inhibitor-resistant cancer cells. Berzosertib is a selective ATR inhibitor. Sacituzumab govitecan consists of an antibody that delivers a chemotherapy payload selectively to certain cancer cells. Researchers want to find out if this drug combination will be effective for people with PARP-resistant tumors and chemotherapy-resistant small cell lung cancer.
Clinicaltrials.gov identifier: NCT04826341
NCI Protocol ID: NCI-00-0-144
Official Title: A Phase I/II Study of Sacituzumab Govitecan Plus Berzosertib in Small Cell Lung Cancer and Homologous Recombination-Deficient Cancers Resistant to PARP Inhibitors
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.