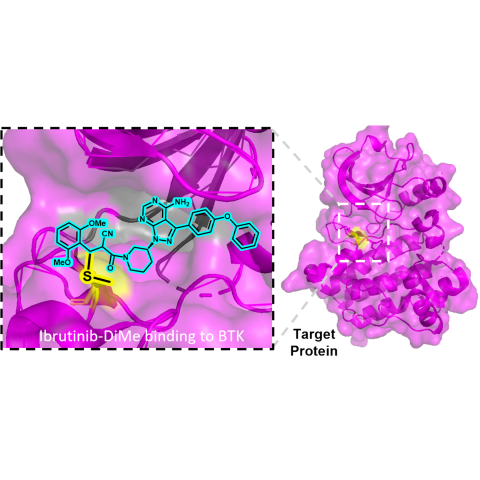
The dimethoxyphenyl cyanoacrylamide (DiMe) chemical warhead facilitates reversible binding of drug to its molecular target. The new warhead is designed to undergo limited hydrolysis, overcoming a common undesirable side reaction for this class of molecules that can complicate their pharmacokinetics and activity.
CCR researchers designed a new chemical component that can be attached to drug molecules to increase their stability in water without losing effectiveness, which could improve the performance of some cancer treatments. The team, led by Joel P. Schneider, Ph.D., Chief and Senior Investigator in the Chemical Biology Laboratory, and postdoctoral fellow Monessha Nambiar, Ph.D., published their discovery on July 9, 2025, in the Journal of the American Chemical Society.
Covalent warheads are components of some drugs that facilitate covalent binding of the drug to its molecular target. These have revolutionized drug development by allowing previously “undruggable” proteins to be targeted. However, these warheads can decompose in the presence of water, potentially lessening their effectiveness.
Schneider’s team serendipitously discovered that the drug voxelotor, when modified with the addition of a chemical group called alpha-cyanoacrylamide, resisted degradation in water. Based on their finding, they designed a new chemical warhead named dimethoxyphenyl cyanoacrylamide (DiMe), which remains stable in water for at least a week while still being able to reversibly interact with protein thiols, an attribute that is crucial for minimizing side effects.
By attaching DiMe to the cancer drug ibrutinib, they were able to make the drug stable in water without losing its effectiveness. Schneider explained that understanding the chemical behavior of this new warhead involved both physical experiments and extensive computational modeling, using resources from CCR and the NCI Frederick campus, particularly the Advanced Biomedical Computational Science group.
The new chemical warhead and the principles learned in this study could have broad use in drug design and development, expanding the utility of these warheads as molecular probes and potentially as new drugs. The Schneider lab is continuing to explore how the DiMe warhead can be used to construct materials for controlled drug release.
Citation: Nambiar M, et al. J Am Chem Soc. 2025 Jul 9;147(27):24140-24151. doi: 10.1021/jacs.5c08170. Epub 2025 Jun 25.
Write-up information provided by: Joel Schneider, Ph.D., and Monessha Nambiar, Ph.D.