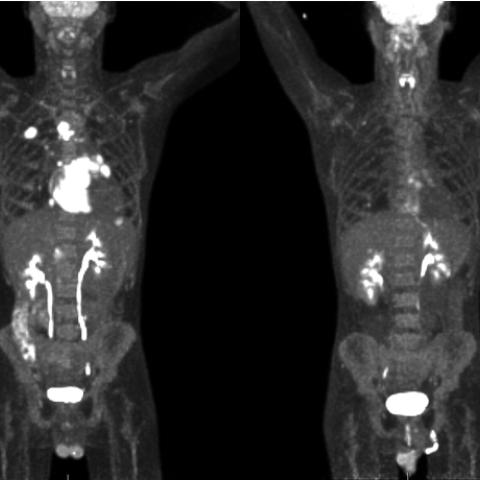
PET scan of patient with virus-associated lymphoma before and after treatment with pomalidomide and anti-PD-1 therapy.
Adults with virus-associated malignancies, including HIV infection, may be eligible to participate in a clinical trial at the NIH Clinical Center.
Several viruses can cause cancer. For example, human papillomavirus can cause cervical and several other cancers, Epstein Barr virus can cause many types of lymphoma, and hepatitis B or C viruses can lead to liver cancer. Viruses can highjack host cells, insert their own DNA or RNA into them, or increase inflammation in the body causing host cells to become cancerous. Less toxic and more effective treatments are urgently needed for cancers caused by viruses. Kathryn A. Lurain, M.D., M.P.H., Assistant Research Physician in the HIV and AIDS Malignancy Branch, is leading a trial of a combination treatment for virus-associated malignancies. Pomalidomide works in several ways to attack cancer cells. It may inhibit the growth of new blood vessels in tumors, boost the action of the immune system, or decrease factors that contribute to tumor growth. Nivolumab is an immune checkpoint inhibitor. T cells can attack and kill certain cancer cells, but cancer cells can evolve proteins to protect themselves from T cells. Nivolumab blocks those protective proteins so T cells can do their work. The goal of the study is to determine the safety and effectiveness of this combination in participants.
Clinicaltrials.gov identifier: NCT04902443
NCI Protocol ID: NCI-21-C-0023
Official Title: A Phase I Study of Pomalidomide and Nivolumab in Patients With Virus-Associated Malignancies With or Without HIV
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.