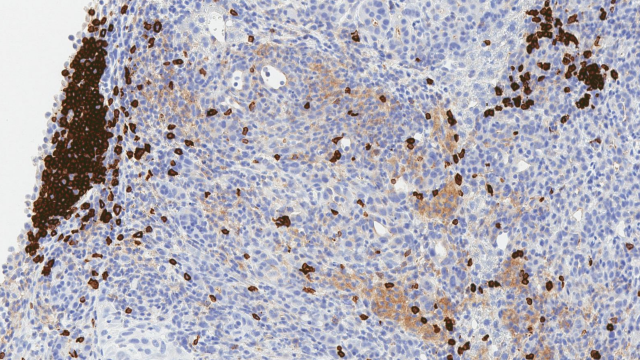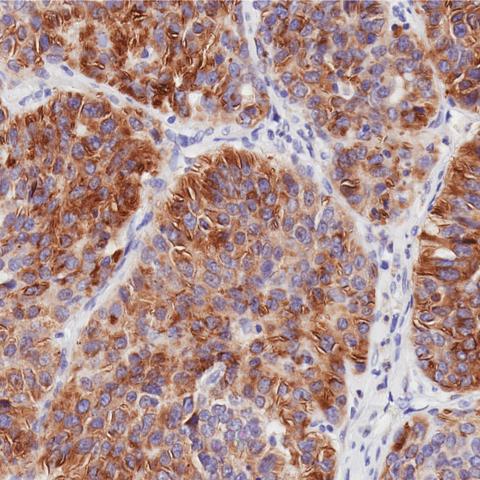
GPC3 expression in hepatocellular carcinoma as determined by immunohistochemistry.
Image courtesy of Dr. Mitchell Ho.
Adults diagnosed with hepatocellular carcinoma (HCC ) may be eligible to participate in a phase I clinical trial that takes advantage of a cell surface protein, called GPC3, that is found on most HCC tumor cells, and importantly, is absent on normal liver tissue and in other organs. The trial is led by Tim F. Greten, M.D., Deputy Chief of the Thoracic and GI Malignancies Branch and co-Director of the NCI CCR Liver Cancer Program.
HCC is the most common type of liver cancer and is a leading cause of cancer-related deaths worldwide. It is notoriously difficult to treat in its advanced stages. Underlying liver disease, such as cirrhosis, is prevalent among patients with HCC and often complicates treatment options. With advanced disease, patients have a median survival of approximately one to two years or less.
One of the major barriers in HCC treatment is finding a way to target cancer cells without causing additional harm to healthy liver tissue. Using an approach called chimeric antigen receptor (CAR) T-cell therapy, Dr. Greten can engineer the immune system to target and eliminate the GPC3-harboring cancer cells. Cancer-fighting T cells (specific white blood cells) are isolated from the participant’s blood and genetically engineered to create antibodies that recognize GPC3. Once enough of the engineered T cells are made, the trial participant undergoes chemotherapy to deplete their own immune system and then receives an infusion of the engineered cells. The goal is for the engineered T cells to recognize and destroy the GPC3-expressing tumors. At the same time, the engineered T cells should proliferate, help repopulate the immune system and continue to eliminate the cancer.
Dr. Greten’s clinical trial builds upon preclinical studies at CCR led by Mitchell Ho, Ph.D., Deputy Chief of the Laboratory of Molecular Biology and Director of the NCI CCR Antibody Engineering Program. Dr. Ho’s lab is responsible for identifying GPC3 in HCC tumor cells and using engineered human T cells to treat human HCC tumors in mice. Dr. Ho’s work, which spans the last decade, set the stage for Dr. Greten’s current clinical trial.
“It’s very rare to find a good tumor-specific target that is highly expressed on the cell surface and is shared by many cancer patients,” says Dr. Ho. In agreement, Dr. Greten adds, “We now have the opportunity to create a potent treatment for a solid tumor, and it was developed from the beginning at CCR.”
Clinicaltrials.gov identifier: NCT05003895
NCI Protocol ID: NCI-21-C-0030
Official Title: Phase I Study of GPC3 Targeted CAR-T Cell Therapy in Advanced GPC3 Expressing Hepatocellular Carcinoma (HCC)
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.