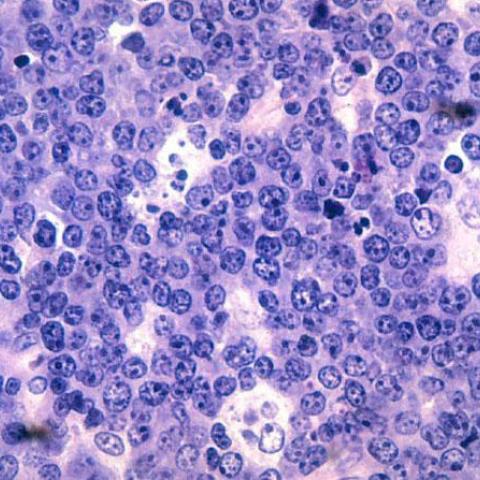
Malignant B-cell lymphocytes seen in Burkitt lymphoma, stained with hematoxylin and eosin (H&E) stain.
Image Credit: NCI Visuals Online
Adults with relapsed or refractory B-cell lymphomas may be eligible to participate in a clinical trial at the NIH Clinical Center.
Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL) are fast-growing cancers that affect B lymphocytes, white blood cells that fight infections and are part of the lymphatic system. BL and DLBCL can also involve the bone marrow, central nervous system and other organs. Initial chemotherapy effectively treats 80–85 percent of adults with BL and 60–70 percent of adults with DLBCL. Those whose disease is not curative or comes back after treatment often have a poor prognosis. Mark Roschewski, M.D., Clinical Director of the Lymphoid Malignancies Branch, is conducting a clinical trial to see if a combination of drugs can help adults with these aggressive diseases. The goal is to see if it is safe to give people with BL and DLBCL copanlisib together with combination chemotherapy.
Clinicaltrials.gov identifier: NCT04933617
NCI Protocol ID: NCI000193
Official Title: Phase 1 Study of Copanlisib With Dose-Adjusted EPOCH-R in Relapsed and Refractory Burkitt Lymphoma and Other High-Grade B-cell Lymphomas
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.