News and Events
Proteins Released from the Nuclei of Dying Cancer Cells Promote Tumor Growth
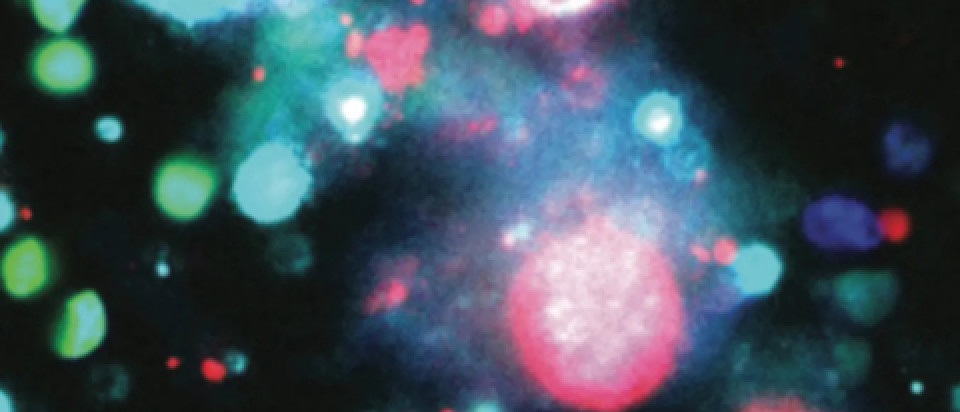
Material released from dying cancer cells, known as tumor cell nuclear expulsion products (TuNEPs), contains specific proteins that promote the growth of neighboring cancer cells. Targeting these proteins could lead to new treatments that hinder cancer spread and improve patient outcomes.
Read MoreClinical trial researching CAR T-cell therapy for acute myeloid leukemia
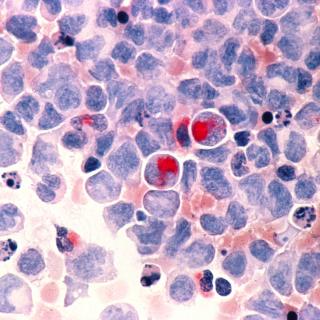
A clinical trial led by Nirali N. Shah, M.D., M.H.Sc., Lasker Clinical Research Scholar in the Pediatric Oncology Branch, is studying the use of CAR T-cell therapy for adult patients with relapsed or refractory acute myeloid leukemia (AML) after an allogeneic hematopoietic stem cell transplant.
Read MoreDietary glutamine may be linked to B-cell lymphomas in abdominal lymph nodes
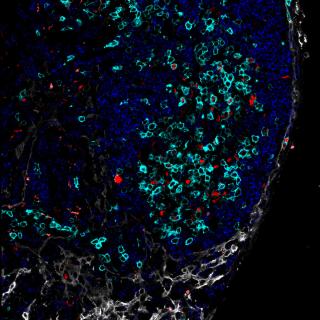
Study provides evidence for different anatomic locations being linked to different biology in lymphoma.
Read MoreRuth Nussinov elected to the European Molecular Biology Organization
Ruth Nussinov, Ph.D., Senior Investigator in the Cancer Innovation Laboratory, was elected as a member of the European Molecular Biology Organization (EMBO). EMBO is an organization of more than 2,000 leading researchers that promotes excellence in the life sciences in Europe and beyond. Election as EMBO Member recognizes a scientist’s research excellence and outstanding achievements in their field.
Read MoreCelebrating CCR Careers: R. Andrew Byrd, Ph.D.
32 years ago, R. Andrew Byrd, Ph.D., came to NCI Frederick to study the foundational biophysics of cancer. After an extensive career developing and utilizing nuclear magnetic resonance (NMR) spectroscopy methods to determine protein structures and mechanistic insight, he has announced his retirement.
Read MoreTechnique targeting cell marker boosts treatment options for B-cell lymphomas
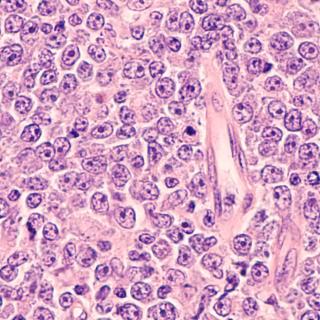
Researchers uncovered the mechanisms behind a drug treatment for diffuse large B-cell lymphoma (DLBCL). The findings help clarify why certain patients respond well to the treatment, while others do not.
Read MoreCelebrating CCR Careers: Terri Armstrong, Ph.D., ANP-BC
Terri S. Armstrong, Ph.D., ANP-BC, FAAN, FAANP, has more than 30 years of experience caring for people with brain and spine tumors. After almost a decade in the Neuro-Oncology Branch, she is announcing her retirement.
Read MoreNew immunotherapy approach shows potential in some people with metastatic solid tumors, NIH researchers say
Early findings from a small clinical trial provide evidence that a new cellular immunotherapy approach may be effective in treating metastatic colorectal cancer. In the trial, researchers led by Steven A. Rosenberg, M.D., Ph.D., Chief of the Surgery Branch, genetically engineered lymphocytes (a type of white blood cell) from each patient to produce receptors that recognize and attack their specific cancer cells. The personalized immunotherapy shrank tumors in several patients and was able to keep the tumors from regrowing for up to 7 months.
Read MoreClinical trial researching combination immunotherapy for endometrial cancer
A trial led by Hoyoung M. Maeng, M.D., Associate Research Physician in the Vaccine Branch, is studying the use of combination drug and vaccine therapy for certain patients with HER2-expressing endometrial cancer.
Read MoreRepairing DNA that breaks during replication
DNA replication can generate double-strand DNA breaks. CCR scientists clarify how these breaks form and how cells repair the potentially catastrophic damage.
Read MoreClinical trial researching immunotherapy for prostate cancer
A trial led by Ravi A. Madan, M.D., Senior Clinician in the Genitourinary Malignancies Branch, is studying hormone blocking therapy and immunotherapy for prostate cancer.
Read More