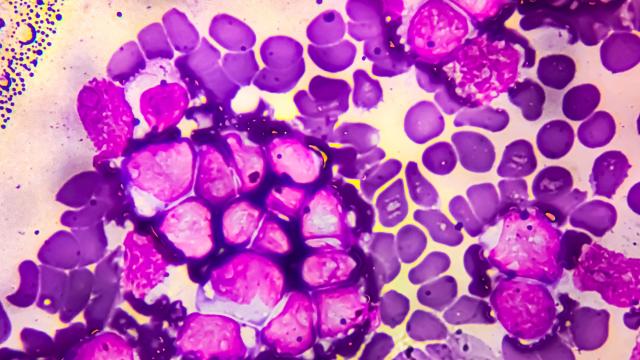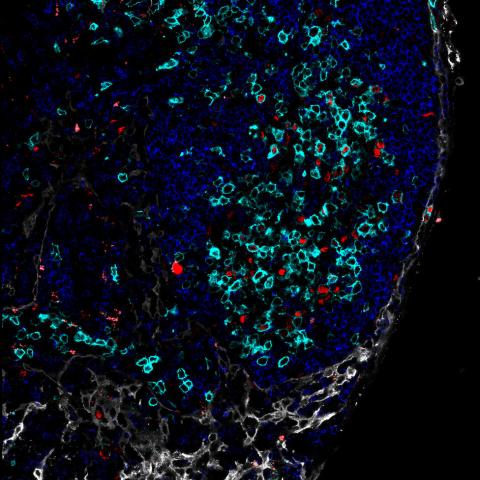
B cells migrating outside of the center of the B cell follicle in mice without GNA13. Image provided by Jagan Muppidi, M.D., Ph.D.
Researchers have discovered that dietary glutamine, an amino acid found in many foods, may be linked to the development of certain kinds of B-cell lymphomas in abdominal structures called mesenteric lymph nodes in mouse models. The study appeared July 18, 2024, in Nature Immunology.
B-cell lymphoma is a common form of cancer of the lymphatic system. It can originate from B cells at different stages of activation. These diverse origins may contribute to how patients respond to therapies.
“One of the field’s bigger efforts over the last 20 years has been trying to define what targeted therapies might be effective in different forms of this common lymphoma,” said Jagan R. Muppidi, M.D., Ph.D., Stadtman Investigator in the Lymphoid Malignancies Branch, and the lead investigator of the study.
The investigators focused on germinal center B cells. These cells are responsible for making antibodies in germinal centers, which are clusters of immune cells that form in lymph nodes. Patients with cancers that are derived from germinal center B cells commonly have loss of function mutations in a gene called GNA13, which encodes the protein G-alpha-13. This protein typically inhibits migration of germinal center B cells.
The researchers used mouse models that lacked the G-alpha-13 protein in B cells and found that these mice tended to develop lymphoma from germinal centers within mesenteric lymph nodes in the abdomen. Additionally, they found gene expression studies showed the absence of G-alpha-13 led to increased activity of a protein called mTOR and increased expression of the protein MYC, both of which are involved in cell growth and proliferation, specifically in germinal center B cells in mesenteric lymph nodes.
“Part of the novelty of our study is that it provides evidence for different anatomic locations being linked to different biology in lymphoma,” said Muppidi. “At present, there are no comprehensive databases that link mutations in lymphoma to the sites of disease, so it isn’t clear whether people with tumors that have GNA13 mutations are more likely to develop disease in the mesenteric lymph nodes as the mice did.”
The researchers next explored possible mechanisms that could explain why the mice developed cancer in this particular location. Within the gut, the lymphatic system transports nutrients such as fats, proteins and vitamins from the intestines to the mesenteric lymph node. Since the G-alpha-13 protein typically confines germinal center B cells to the center of the B cell follicle, researchers hypothesized that these B cells in mice without G-alpha-13 could be migrating outside of the center of the cells and be exposed to substances in the lymphatic system that originated in the gut.
The researchers did discover that this was the case, and specifically found that diet-derived glutamine supported increased levels of MYC and mTOR activity in G-alpha-13-deficient germinal center B cells, leading to increased proliferation.
“We are interested in more completely defining the molecular pathway by which dietary glutamine and potentially other nutrients support the expression of MYC in G-alpha-13-deficient cells,” Muppidi said. These findings are only applicable in mouse models today, but if extended into future human studies, this research could eventually identify therapeutic targets for G-alpha13-deficient lymphoma. Next, the researchers hope to learn whether alteration of dietary glutamine or other dietary modifications can suppress the progression of existing lymphoma in animal models.