Our Discoveries
Machine learning reveals rules of T cell activation
CCR and McGill University scientists have used machine learning to build models that can predict T cell responses against tumors.
Read MoreComputational model finds signatures of T cell resilience in solid tumors
Evaluating T cell resilience can help researchers predict — and possibly improve — immunotherapy outcomes.
Read MoreProtein signature in the blood linked to prostate cancer lethality in men of African ancestry
African American men have an increased risk for lethal prostate cancer. CCR researchers have found that an immunity-related protein signature in the blood is more prevalent in men of African ancestry, which may help explain this devastating health disparity.
Read MoreUnique gene expression profiles identify rare T cells that could be effective in cancer immunotherapy
CCR researchers led by Steven A. Rosenberg, M.D., Ph.D., Chief of the Surgery Branch, have found unique expression profiles in 50 genes that help identify rare anti-tumor lymphocytes that can be used as personalized immunotherapy in many patients with metastatic solid tumors.
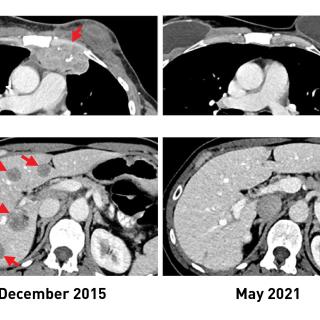
Read MoreNCI study advances personalized immunotherapy for metastatic breast cancer
An experimental form of immunotherapy that uses an individual’s own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer, according to results from an ongoing clinical trial led by Steven A. Rosenberg, M.D., Ph.D., Chief of the Surgery Branch.
Read MoreResearchers develop new method to estimate cell-type-specific information from bulk tumor data
CCR researchers have established a way to extract cell-type-specific expression information from bulk gene expression data of a tumor sample. Their computational approach, which outperforms comparable state-of-the-art methods, evaluates thousands of tumor samples with minimal cost.
Read MoreBreast cancer stem cells become aggressive upon contact with macrophages
New findings suggest that breast cancer cells gain stem-like characteristics of invasiveness, aggressiveness and drug resistance upon contact with macrophages, specialized white blood cells in the immune system.
Read MoreA high-fiber diet may improve the response of melanoma patients to immunotherapy
A new study co-led by Giorgio Trinchieri, M.D., chief of the Laboratory of Integrative Cancer Immunology, found that a diet rich in fiber may help some people being treated for melanoma respond to immunotherapy treatment by influencing the gut microbiome. The new findings come from an analysis of people with melanoma and mouse models of the disease.
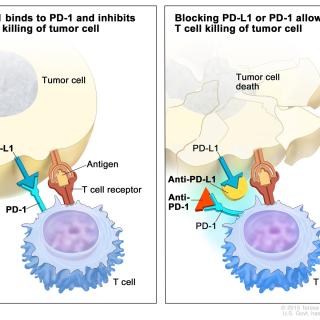
Read MoreCell surface molecule could predict how patients respond to immunotherapy
A new study finds that some immunotherapy drugs are less likely to control cancer in patients who carry HLA-A*03, a cell surface molecule that is involved in immune system responses. This work, which was featured in NCI's Cancer Currents, reveals that HLA-A*03 is a potential biomarker for patient prognosis following certain immunotherapy treatments.
Read MoreCCR researchers use immunogenetic profiling to identify targets for numerous pediatric cancers
Researchers analyzed over 700 pediatric tumor samples and cell lines, creating one of the largest profile datasets of gene expression and immune response in solid pediatric tumors to date. Their work reveals that a germline developmental protein called PRAME is a potential cancer target.
Read More