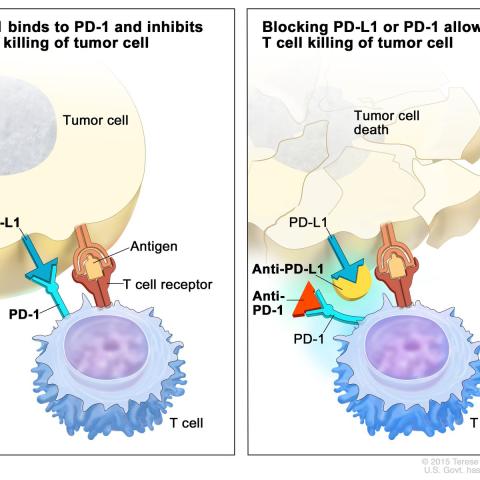
Depiction of immune checkpoint inhibition therapy targeting a cell surface protein called PD-1. Immune checkpoint inhibition blocks the safety signals of PD-1 and allows the immune system's T-cells to recognize tumor cells as foreign. This leads to T-cells attacking and killing tumor cells.
Credit: NCI, Visuals Online
Researchers from CCR found that patients who carry a cell surface molecule called human leukocyte antigen A*03 (HLA-A*03) have a poor response to immune checkpoint inhibition therapy. This collaborative work, published in The Lancet Oncology on Dec. 9, 2021, offers a novel approach to evaluating the utility of immune checkpoint inhibition therapy by using HLA-A*03 as a potential biomarker.
Immune checkpoint inhibition is a form of immunotherapy that enhances the immune system’s ability to see and destroy cancer cells, but while the approach works well for some, immune checkpoint inhibition fails in more than half of patients. To better understand why some patients do not respond to this type of immunotherapy, Mary N. Carrington, Ph.D., senior investigator in the Laboratory of Integrative Cancer Immunology, asked if variations in class I HLA molecules – flags on the cell surface that distinguish between normal and aberrant cells – could predict patient response to immune checkpoint inhibition. These molecules reside on all human cells with a nucleus and present foreign materials to initiate an immune response. But not everyone has the same set of class I HLA variants, which are inherited from each parent and vary widely among people from different ethnic backgrounds.
Carrington reached out to James L. Gulley, M.D., Ph.D., chief of the Genitourinary Malignancies Branch, who had access to groups of cancer patients from his work on the immune checkpoint inhibition drug avelumab.
The researchers examined advanced cancer treatment outcomes in eight groups of patients, including over 3,000 patients treated with immune checkpoint inhibition drugs and over 10,000 patients treated with alternate therapies, and correlated those outcomes with patient HLA variants. In all groups, the presence of at least one copy of HLA-A*03 decreased the length of time before the cancer progressed with immune checkpoint inhibition treatment but not with chemotherapy. The rate of cancer progression was even worse with an additional copy of HLA-A*03. “All of the studies where patients were randomized to immunotherapy versus non-immunotherapy treatments had the same finding. This really adds to the strength of the data and suggests that there is something here,” Gulley says.
Their next goal is to verify this finding in larger groups of patients and determine the mechanism that causes a poor response to immune checkpoint inhibition for HLA-A*03 carrying patients. “If we can understand why HLA-A*03 associates with poor treatment response, we might be able to enhance patient response to immune checkpoint inhibition in general to avoid these negative outcomes,” Carrington says.
Beyond these immediate next steps, this work has implications for clinical trial recruitment to test new immunotherapy drugs. “If we enroll a more diverse group of patients, we are more likely to see a broader representation of HLA types, and this could be important as we come across findings like this,” Gulley says. “It is absolutely critical during clinical trials to get a broader representation so we can pick up on this type of data.”
Read more about this study in the NCI Cancer Currents blog.