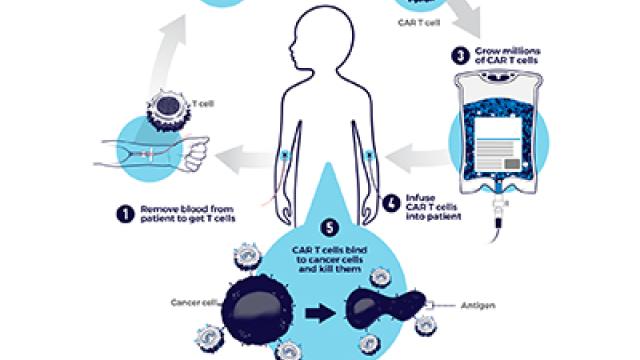
We conduct collaborative clinical trials that focus on developing targeted agents and applying immunotherapy to treat high risk childhood leukemias and lymphomas that do not respond to chemotherapy. Our patients come to us from all over the world to receive treatment when previous therapies have failed. To learn more about our clinical trials, please contact our team.
Leukemia, Lymphoma, Transplant and Cell Therapy Team
Our highly trained team is focused on developing and conducting a wide range of clinical trials in a quest to improve outcomes for children, adolescents, and young adults with leukemias and lymphomas.
Leukemia, Lymphoma, Transplant and Cell Therapy Team
About
Although cure rates are excellent for many subtypes of childhood leukemia and lymphoma, new approaches are needed to overcome resistance to standard therapies and to decrease treatment-associated sequelae in survivors of pediatric cancer.
Our goal, as the Leukemia, Lymphoma, Transplant and Cellular Therapy Team of the Hematologic Malignancies Section, is to develop cutting-edge, early-phase clinical trials for patients with high-risk hematologic malignancies, with a specific focus on developing novel therapies which can overcome chemotherapeutic or immunotherapeutic resistance. The trials implemented by our section seek to improve upon existing strategies, or address unmet needs, and incorporate the study of leukemia biology to optimize outcomes.
Our group is specifically focused on advancing CAR T-cell therapy for children and young adults with acute leukemias and lymphomas. Our focus is on using CAR T-cells to target new tumor markers in acute lymphoblastic leukemia (ALL) and acute myelogenous leukemia (AML) and learn how to best deliver this care safely and effectively. With over a decade worth of experience in treating children and young adults with CAR T-cells and a highly collaborative infrastructure, our team has identified optimal strategies to treat cytokine release syndrome, pioneered novel treatment approaches for CAR T-cell mediated toxicity and embraces a holistic approach to ensure that each patient and family’s individual experiences are incorporated when planning how to optimize care.
Our team is a member of the CARnation Consortium, a group of expert researchers and clinicians treating pediatric hematologic malignancies with immunotherapy.

Patients & Providers
Group
Contact Our Team
240-858-3536 (Lauren Little, RN) or 240-383-6185 (Toni Foley, RN)
NCILLTCT@mail.nih.gov
Clinical Trials
Team
News
CARnation Consortium
Learn about the efforts of the CARnation Consortium, a multi-institution, multidisciplinary collaborative effort encompassing retrospective and prospective studies focused on improving outcomes following CAR T-cell therapy in children and young adults.
10 Years of CAR T-cells in POB
On July 13th, 2022, the POB celebrated the 10 year anniversary of the first patient infused with CD19 CAR T-cells in the Pediatric Oncology Branch. POB staff honored our patients and families and gave talks on key research advancements and milestones in immunotherapy research. Dr. Steve Rosenberg gave the keynote address, "The History of CD19 CAR."
Watch a recording of the symposium here.
CAR T-cell Therapy: Beyond the Storm - May 2020
Held virtually in May 2020, this conference served to bring together leading institutions and investigators focused on pediatric CAR T-cell efforts for an information exchange to specifically focus on other elements of CAR T-cell toxicities, beyond cytokine release syndrome (CRS). One aim was to highlight the biologic underpinnings of various elements of the extended toxicity profile exhibited in patients and compile current approaches and best practices utilized in toxicity mitigation and management strategies. Furthermore, with novel antigens and CAR T-cell targets, this conference helped to establish standardized metrics of monitoring for these comprehensive toxicities as we go beyond B-cell targeting. This conference served to lay the foundation of how to best study these extended outcomes and optimize care for patients receiving CAR T-cell therapy, beyond the storm.
Read the resulting publication: Shalabi H., et al. “Beyond the storm - subacute toxicities and late effects in children receiving CAR T cells.” Nat Rev Clin Onc. 2020.
Science
Current research activities:
- Development of anti-CD19, anti-CD22 and anti-CD33 chimeric antigen receptor (CAR) therapy and other targeted agents and immune-based therapies
- Novel approaches to hematopoietic stem cell transplantation, with a focus on immune restoration and treatment of patients with primary immune deficiencies
- Post-transplant allogeneic immunotherapy to direct anti-tumor responses and treatment of relapse after transplant
- Bedside-to-bench based studies designed to support current and future clinical trials
Collaborative research efforts include studies on:
- CAR-T cell toxicity
- Neurotoxicity
- Infectious disease
- Patient reported outcomes
- Late effects
- Antigen modulation
- Stem cell transplant
- Education of minors who donate stem cell
Recent Press and Publications:
- Listen: NPR Health News Article - Scientists Race to Improve 'Living Drugs' to Fight Cancer
- Watch: The Role of Immunotherapy in Childhood Cancer Treatment
- Watch: Moving Therapies from the Relapse/Refractory Setting to the Frontline
- Watch: 2018 NIH Demystifying Medicine Lecture: Is Childhood Leukemia Curable?
- Virtual Meeting: CAR-T Cell Therapy: Beyond The Storm
- Read: Bispecific CD19/CD22 CAR T Cells Active in Pediatric Relapsed ALL
- Read: NCI Initiative Aims to Boost CAR T-cell Therapy in Clinical Trials
- Read: Near the Nation's Capitol Lies a 'House of Hope'
Contact
Call one of our nurses:
Toni Foley, RN 240-383-6185
Or reach us by email:
We will get in contact with you and your medical team to collect all of the necessary medical records and discuss the next steps of meeting with our team at the NIH Clinical Center.
CAR T-cell Program
The CAR T-cell Experience in the Pediatric Oncology Branch: For Patients and Families
We understand that it can be stressful coming to a new institution and meeting with an entirely new group of people. We want you to know that we are here to support you and your family. Whether you are coming to get your first CAR T-cell ever, or you have received CAR T-cells before, our team is here to answer your questions. We are committed to providing comprehensive psychosocial support to you and your family throughout your care at NIH.
In addition to assistance with housing, access to food and transportation, psychosocial services include social work, psychology, neuropsychology, psychiatry, palliative care, recreation therapy, art therapy and chaplaincy. The NIH also has its own school to instruct school-age children and to help them keep up with their academic needs.
Key collaborators and colleagues include:
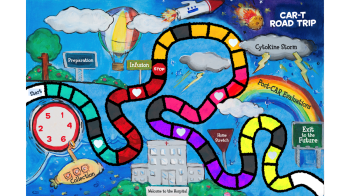
Our efforts have included the development of a CAR T-cell game which we can play with children to explain the CAR T-cell process.
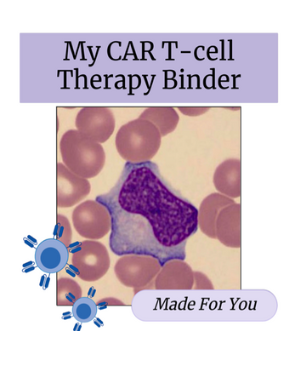
You will receive a binder to help keep track of key information and write down any questions that you may have for your team!
We are very committed to optimizing psychosocial care for children receiving CAR T-cell therapy. Please see our publication on this work here. We also have studies ongoing to evaluate the patient and caregiver experience.
The Pediatric Oncology Branch is as committed to the emotional wellbeing of our patients and families as we are to the state-of-the-art (or exceptional) medical care provided. Psychosocial support is available to all patients and their caregivers and tailored to individual needs and preferences. In addition to assistance with housing, access to food and transportation, psychosocial services include social work, psychology, neuropsychology, psychiatry, palliative care, recreation therapy, art therapy and chaplaincy. The NIH also has its own school to instruct school-age children and to help them keep up with their academic needs. Learn more about our psychosocial support program here.