CAR T-cell therapy
Image courtesy of NCI Visuals Online
Children and young adults with acute myeloid leukemia that has progressed after initial treatment may be eligible to participate in a new clinical trial at the NIH Clinical Center.
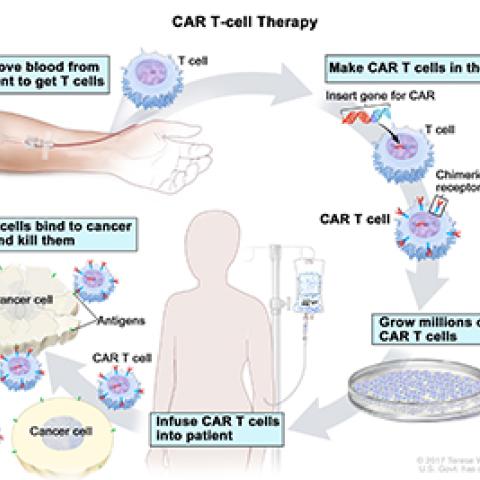
Nirali Shah, M.D., M.H.Sc., Lasker Clinical Research Scholar in the Pediatric Oncology Branch, is leading NCI’s participation in a trial of CAR T-cell therapy for acute myeloid leukemia (AML) that has progressed after initial treatment. AML is a cancer of the blood and bone marrow, the soft inner part of long bones where new blood cells are made. With AML, bone marrow cells don't grow the way they're supposed to. Instead, immature bone marrow cells grow uncontrollably and build up in the body. This study is testing the effect of chimeric antigen receptor T cells (CAR T cells) in AML. CAR T cells are T cells that have been genetically engineered to give T cells the ability to attack cancer cells. T cells are taken from a patient's blood, and a special receptor that binds to the patient's cancer cells is added to the T cells in the laboratory. Large numbers of CAR T cells are grown then given back to the patient by infusion. The first part of the study will determine the safest dose of CAR T cells. The second part will determine how well patients respond to this therapy.
Clinicaltrials.gov identifier: NCT03971799
NCI Protocol ID: NCI-20-C-0028
Official Title: Phase 1/2 Study of Anti-CD33 Chimeric Antigen Receptor-Expressing T Cells (CD33CART) in Children and Young Adults With Relapsed/Refractory Acute Myeloid Leukemia
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.