News and Events
Proteins Released from the Nuclei of Dying Cancer Cells Promote Tumor Growth
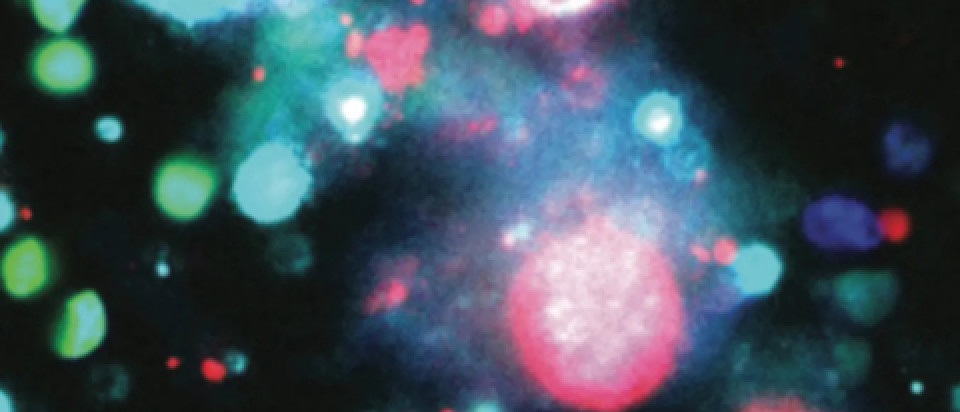
Material released from dying cancer cells, known as tumor cell nuclear expulsion products (TuNEPs), contains specific proteins that promote the growth of neighboring cancer cells. Targeting these proteins could lead to new treatments that hinder cancer spread and improve patient outcomes.
Read MoreNew CAR T-cell therapy eliminates rhabdomyosarcoma in mice
After seeing excellent results of a CAR T-cell therapy against rhabdomyosarcoma in mice, a team of CCR researchers is preparing to test the immunotherapy in humans.
Read MoreCCR Hosts “Frontiers in Basic Immunology” Symposium
CCR hosted a two-day international symposium, titled “Frontiers in Basic Immunology,” sponsored by the NCI Center of Excellence in Immunology (CEI). The program included a lineup of presentations from NCI and worldwide academic university speakers and a lively poster session.
Read MoreClinical trial researches radiation therapy for prostate tumors
A clinical trial led by Krishnan R. Patel, M.D., Assistant Research Physician in the Radiation Oncology Branch, is researching radiation therapy for prostate cancer.
Read MoreThe ups and downs of a rare blood cancer won’t stop one resilient patient
Even with a rare cancer diagnosis and a roller-coaster ride of a journey, Luis Miguel Cruz continues to find the motivation to keep moving forward through every setback. Along the way, he has inspired and brought joy to those he has met, both in person and digitally.
Read MoreClinical trial researches targeted therapy for biliary tract cancers
A clinical trial led by Jonathan M. Hernandez, M.D., Investigator in the Surgical Oncology Program, is researching targeted therapy for cholangiocarcinoma and gallbladder cancer.
Read MoreCCR researchers receive an array of HHS awards
Three CCR researchers received prestigious awards from the Department of Health and Human Services on September 28, 2023. They are being recognized for their excellence, dedication and achievements in their respective research fields.
Read MoreClinical trial researches focal therapy for prostate cancer
A clinical trial led by Peter A. Pinto, M.D., Senior Investigator in the Urologic Oncology Branch, is researching guided focal therapy for prostate cancer.
Read MoreClinical trial researches combination drug therapy for kidney cancer
A clinical trial led by Ramaprasad Srinivasan, M.D., Ph.D., Senior Investigator in the Urologic Oncology Branch, is researching combination drug therapy for certain renal cell carcinomas.
Read MoreClinical trial researches CAR T-cell therapy for B-cell cancers
A clinical trial led by James N. Kochenderfer, M.D., Senior Investigator in the Surgery Branch, is researching CAR T-cell therapy for B-cell leukemias and lymphomas.
Read MoreClinical trial researches antibiotic therapy for lung cancer
A clinical trial led by Chen Zhao, M.D., Stadtman Investigator in the Thoracic and GI Malignancies Branch, is researching a combination therapy for non-small cell lung cancer.
Read More