Clinical Trials
FDA approves belzutifan, first drug for cancers associated with von Hippel-Lindau disease
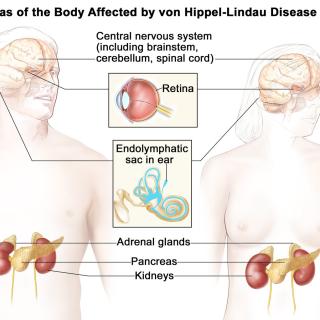
On August 13, 2021, the Food and Drug Administration approved belzutifan, a new drug for adult patients with von Hippel-Lindau (VHL) disease-associated renal cell carcinoma (RCC), central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors, not requiring immediate surgery.
Ramaprasad Srinivasan, M.D., Ph.D., Investigator in the Urologic Oncology Branch (UOB), designed the ongoing study and played a key leadership role as the principal investigator on the cooperative research and development agreement under which NCI served as a site in the study. Belzutifan is now the first and only approved systemic therapy for certain patients with VHL-associated RCC.
VHL disease is a rare, inherited disorder that causes tumors and cysts to grow in certain parts of the body. Patients with this disease have an increased risk of certain types of cancer, especially kidney cancer and pancreatic cancer. The VHL gene was originally identified by Marston Linehan, M.D., and colleagues in the UOB in the 1990s, and the group continues to define the methods for clinical management of VHL disease.
Read MoreNew study tests drug combination for treatment of biliary tract cancer
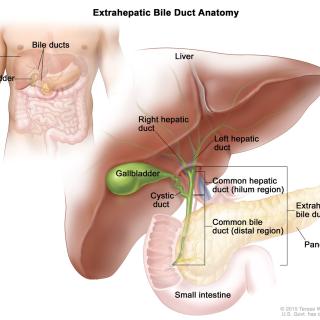
Biliary tract cancer (BTC) is a cancer that arises from the bile ducts that carry bile, a digestive fluid, through the liver. There are few treatment options for BTC. Tim F. Greten, M.D., Deputy Chief of the Thoracic and GI Malignancies Branch, is leading a study of a drug combination that may prolong survival in adults with BTC.
Read MoreClinical trial investigates drug combination for relapsed small cell lung cancers and advanced neuroendocrine cancers
Anish Thomas, M.B.B.S., M.D., Lasker Clinical Research Scholar in the Developmental Therapeutics Branch (DTB), and Jaydira Del Rivero, M.D., Assistant Research Physician in DTB, are heading a trial testing the safety and efficacy of berzosertib, an ATR inhibitor, in combination with lurbinectedin to treat relapsed small cell lung cancer or high-grade neuroendocrine cancers.
Read MoreClinical trial will test triple-drug combination against aggressive colon and HPV-associated cancers
CCR researchers are exploring whether a special cocktail of drugs, which collectively make cancer cells vulnerable and the immune system stronger, will yield better outcomes for patients with difficult-to-treat cancers. Preliminary research in preclinical models suggests that the triple-drug combination is more effective than just one or two of the drugs being administered.
Read MoreCollaborative study leads to FDA approval of belumosudil for chronic graft-versus-host disease
The Food and Drug Administration (FDA) approved belumosudil on July 16, 2021, for people 12 years and older with chronic graft-versus-host disease (cGVHD) after failure of at least two prior lines of systemic therapy. Chronic GVHD is a complex condition that can be life threatening and occurs when donated stem cells attack healthy tissues in a patient’s body. Steven Z. Pavletic, M.D., M.S., Senior Clinician in the Immune Deficiency Cellular Therapy Program, guided CCR’s involvement in the pivotal consortium study that led to FDA approval of belumosudil. Pavletic was part of the trial clinical leadership at CCR, one of the 28 centers that enrolled study patients. The study found belumosudil to be safe and well-tolerated, and it may have the potential to improve overall patient well-being.
Read MoreClinical trial studies six-drug combination aimed at relapsed/refractory B-cell lymphoma
Christopher J. Melani, M.D., Assistant Research Physician in the Lymphoid Malignancies Branch, is leading a study to see whether a combination of six drugs is safe and can help people with both aggressive and indolent B-cell lymphoma.
Read MoreNew study tests therapy in patients with hemorrhagic cystitis after bone marrow transplant
Jennifer A. Kanakry, M.D., Associate Research Physician in the Experimental Transplantation and Immunotherapy Branch, is leading CCR’s participation in a study of a drug, Viralym-M, that targets six viruses that can cause hemorrhagic cystitis (HC) in people who have had a hematopoietic cell transplant. Researchers are most interested in Viralym-M’s ability to clear the symptoms of HC faster than a placebo and evaluating Viralym-M’s effect on five other viruses.
Read MoreClinical trial studies immunotherapy and radiation for pancreatic cancer
Fewer than 10 percent of people with pancreatic cancer can have surgery, which offers the best chance of a cure. Udo Rudloff, M.D., Ph.D., Senior Investigator in the Pediatric Oncology Branch, is leading a study testing a combination of immunotherapy and radiation for people with advanced pancreatic cancer to shrink tumors and make surgery possible.
Read MoreA conversation with CCR immunotherapy experts
June is Immunotherapy Awareness Month. We asked members of the Surgery Branch about their work with immunotherapy as they continue to make important advances in this growing field.
Read MoreClinical trial evaluates COVID-19 vaccine in adults in treatment for cancer
Adults with hematologic malignancies or solid tumors may be eligible to participate in a new clinical trial at the NIH Clinical Center. Elad Sharon, M.D., M.P.H., Senior Investigator at the National Cancer Institute, is leading a study to assess the effects of the Moderna COVID-19 vaccine (mRNA-1273) in adults undergoing treatment for various types of cancer.
Read More