Carol J. Thiele, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 10 - Hatfield CRC, Room 1W- 3940 (office)
- Bethesda, MD 20892-1928
- 240-858-3849
- thielec@mail.nih.gov
RESEARCH SUMMARY
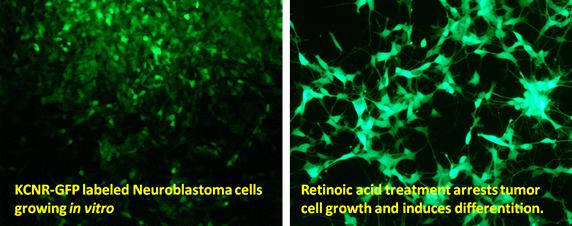
Dr. Thiele leads a research program which develops novel therapies for children with solid tumors using state-of-the-art biologic and genomic analyses of tumors and normal counterparts. She pioneered studies using retinoids to “target” the MYCN oncogene and control tumor growth. These led to clinical studies which showed that retinoids improved outcomes for children with high-risk neuroblastoma.
Her section has developed pre-clinical models and genetically engineered mice (GEMs) to study mechanisms of neuroblastoma tumorigenesis and assess novel therapeutic interventions. Ongoing studies are aimed at understanding epigenetic/chromatin based mechansims to re-program and differentiate neuroblastoma tumor cells.
Areas of Expertise

Carol J. Thiele, Ph.D.
Research
Cell and Molecular Biology Section
Every year approximately 700 cases of neuroblastoma (NB) and 210 cases of Ewings' sarcoma (EWS) are diagnosed in children less than 20 years of age. Half will fail conventional therapy. Despite the progress made in the development of clinical/genetic-based staging systems for NB and EWS the prognosis for patients with unfavorable disease remains dismal. The focus of the Cell and Molecular Biology Section has been to develop a comprehensive understanding of the biology of peripheral neuroectodermal tumors cells. We do so by developing in vitro and in vivo modeling systems that will enable genetic interrogation of the biologic systems of tumor cells and their normal counterparts. The insights gained from these studies serve as a platform for the development of novel therapies for the treatment of children with these diseases.
A number of genetic alterations have been identified in neuroblastoma tumors which are thought to contribute to tumorigenicity. Despite these genetic alterations, retinoids (derivatives of Vitamin A) are capable of arresting cell growth, inducing differentiation and suppressing tumorigenicity. Thus by activating alternative intracellular signaling programs we may be able to bypass genetic defects and restore growth control and induction of differentiation.
Project I: The BDNF/TrkB/PI3-Kinase/AKT Pathway Is Important in the Survival and Metastatic Capability of NB Tumor Cells.
The specific aims of this project are:
- To identify and characterize the molecular mechanisms mediating these processes; and
- To evaluate the therapeutic potential of targeting downstream signaling intermediaries of the PI3-Kinase/AKT pathway to enhance chemosensitivity and inhibit metastasis and angiogenesis.
Project II: Clinical, Histopathologic and Genetic Evidence Indicates that Alterations in the Regulation of Normal Development Contribute to NB Tumorigenesis.
The specific aims of this project are:
- To identify and characterize NB tumor-initiating cells/cancer stem cells;
- To identify the epigenetic pathways dysregulated in NB by using chemical and siRNA screens to identify comounds that target these epigenetic pathways; and
- To study the epigenetic mechanisms regulating NB differentiation by using chemical and small molecule screens to identify agents that stimulate differentiation.
Publications
- Bibliography Link
- View Dr. Thiele's PubMed Summary.
Targeting SWI/SNF ATPases reduces neuroblastoma cell plasticity
CASZ1 induces skeletal muscle and rhabdomyosarcoma differentiation through a feed-forward loop with MYOD and MYOG
Targeting the Chromosomal Passenger Complex Subunit INCENP Induces Polyploidization, Apoptosis, and Senescence in Neuroblastoma
Lineage specific transcription factor waves reprogram neuroblastoma from self-renewal to differentiation
Epigenetic siRNA and Chemical Screens Identify SETD8 Inhibition as a Therapeutic Strategy for p53 Activation in High-Risk Neuroblastoma
Biography

Carol J. Thiele, Ph.D.
Dr. Thiele received her Ph.D. in Microbiology and Immunology from the University of California, Los Angeles. She completed her postdoctoral research as a Cancer Research Institute and a Damon Runyon-Walter Winchell Fellow at the NCI. Dr. Thiele was one of the founding editors of Cell Death & Differentiation, and has served on the editorial boards of Cell Death & Differentiation, Cancer Research and Molecular Cancer Therapeutics. Dr. Thiele was Chair of the AACR Women in Cancer Research and has a long-standing interest in developing programs so that young scientific investigators can realize their potential. As the Chief of the Cell and Molecular Biology Section in the Pediatric Oncology Branch, Dr. Thiele's scientific interest is in the field of cancer biology with a special emphasis on pediatric neuroectodermal tumors and neuronal development. She has been involved in the organization of the Advances in Neuroblastoma Research Association (ANRA). Her research strives to understand molecular mechanisms involved in the pathogenesis of neuroblastoma tumors and utilizes insights gleaned from these studies to develop novel therapeutic strategies for pediatric tumors.
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
More News from Our Lab
2023
Director's Innovation Award
- Zhihui Liu, Ph.D.
2021
CDMRP Award
- Zhihui Liu, Ph.D., Single-Cell CRISPRa Screen to Identify Transcription Factors That Mediate Neuroblastoma Phenotypic Switching and Chemotherapy Drug Resistance
Director's Innovation Award - Career Development
- Zhihui, Liu, Ph.D., Hi-C-based identification of chromatin associated proteins and eRNAs
2017
AACR Aflac Scholar-in-Training Award
- Veronica Veschi, M.D., Ph.D.
17th CCR-FYI Colloquium - Outstanding Oral Presentation
- Veronica Veschi, M.D., Ph.D.
2016
Advances In Neuroblastoma Research 2016 – Best Abstracts
- Deblina Banerjee - Reactivation of cAMP PKA pathway is an early even that relieves EZH2-mediated epigenetic suppression in High-Risk Neuroblastoma (HR-NB)
2014
Advances In Neuroblastoma Research 2014 – Best Abstracts
- Carol Thiele, Zhihui Liu - Whole Genome Screen to identify genes targeting MYCN driven embryonic tumors - Neuroblastoma model
Lab Life

Eclipse 2024 as seen from Stefano at the CMBS Eclipse watch event.
2024 Lab Picture

CMBS Traditional Cherry Blossom Picnic, 2024

Zhihui, Wendy, Xingyu, and Kaitlyn at Postbac Poster Day 2024