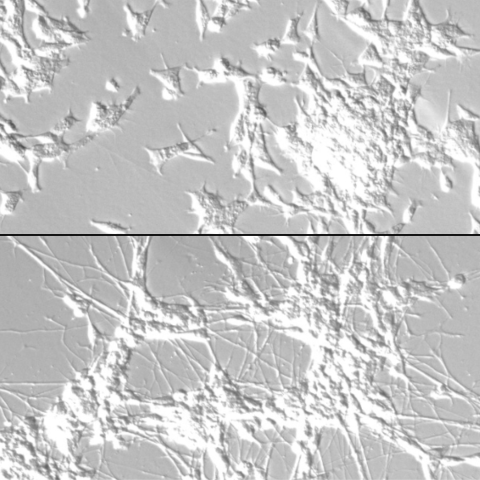
Neuroblastoma cells develop from neuronal progenitor cells that are stuck in a pattern of self-renewal (top). CCR researchers identified master regulators that can reprogram the cells and drive them to develop into neurons (bottom), which halts the tumor cell proliferation. Image: Thiele lab
CCR scientists have mapped key regulatory changes that occur when a common neuroblastoma therapy, retinoic acid, drives tumor cells to develop into neurons, thereby curbing their growth. The findings, published April 23, 2024, in Nature Communications, reveal the loss of regulatory elements that maintain neuroblastoma cells’ potential to divide, and the gain of elements that promote their maturation into neurons. The findings also clarify the effects of using retinoic acid to treat neuroblastoma, a pediatric cancer that begins in the peripheral nervous system.
As a cancer of the peripheral nervous system, neuroblastoma develops in parts of the body outside the brain and spinal cord (which make up the central nervous system) — most commonly in or around the adrenal glands, which are located on top of the kidneys. Carol J. Thiele, Ph.D., Deputy Chief of the Pediatric Oncology Branch, explains that neuroblastoma growth appears to be driven by disruption of the developmental pathways that control the cells that give rise to certain kinds of peripheral neurons. Mutations in genes that control regulatory circuits trap the immature cells in a pattern of self-renewal, spurring continuous cell division rather than permitting the cells to develop into neurons.
Retinoic acid, a derivative of vitamin A, can reprogram neuroblastoma cells and cause them to develop into neurons, a process known as differentiation. This has prompted Thiele and others to wonder about the pathways that control the cells’ developmental state.
“The fact that a substance like vitamin A can reimpose growth control and induce a differentiation program means that even though mutations are dysregulating neuroblastoma cells, they can still be controlled,” says Thiele. The investigators knew that such a dramatic transformation would involve changes in the activity of many genes and wanted to find the master regulators that coordinate that process.
To uncover the relevant transcriptional circuitry, Thiele’s team worked with colleagues in the lab of Javed Khan, M.D., Deputy Chief of the Genetics Branch, to catalog changes throughout the genome of lab-grown neuroblastoma cells at various times after they were treated with retinoic acid. The effort was led by postdoctoral researchers Deblina Banerjee, Ph.D., and Berkley Gryder, Ph.D.
The alterations they found occurred in waves. Within days of being exposed to retinoic acid, neuroblastoma cells lost regulatory elements called super-enhancers on genes that power their self-renewal. Then, after a wave of transient changes, the cells acquired different super-enhancers on genes that promoted their differentiation.
Super-enhancers influence the activity of sets of genes by switching on the production of gene-activating proteins called master transcription factors. Thiele and her team identified several master transcription factors that were key to neuroblastoma cells’ differentiation. These included self-renewal-promoting transcription factors like SOX11, which were lost early in the process, as well as differentiation-promoting transcription factors like SOX4, which appeared a few days later.
The findings give a clearer picture of how retinoic acid treatment benefits patients with neuroblastoma and hint at why certain subtypes of the cancer are more responsive to the therapy than others. By digging deeper into the new findings, researchers may be able to identify new ways to reprogram neuroblastoma cells to stop their growth.