News and Events
New Strategy Shows Promise Against Deadly Breast Cancer in the Brain
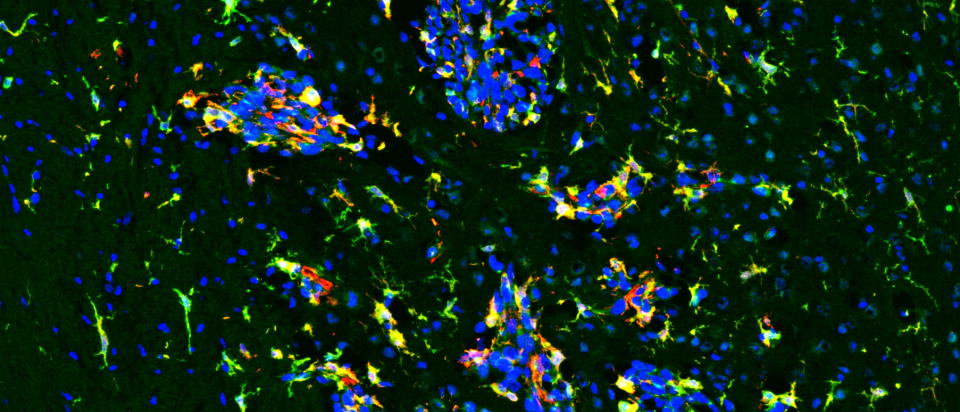
A new NIH study points to a promising strategy for treating aggressive breast cancer that spreads to the brain, a complication with few effective options. Learn how blocking a key brain cell survival pathway could open the door to future therapies.
Read MorePatient Testimonials
Read testimonials from patients and their families about their time at CCR.
Read MoreActivated gene elicits robust immune response in lung cancer
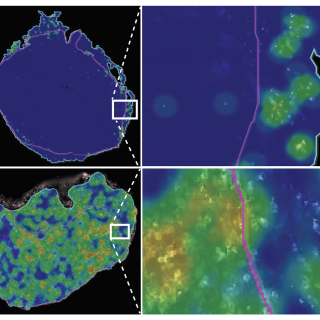
The activation of NOTCH1, a gene known to control cell differentiation and growth, stimulates a robust immune response in small cell lung cancer. Expression of this gene can also predict survival with immunotherapy.
Read MoreStudy uncovers new details on pulsing enzyme activity during cell cycle
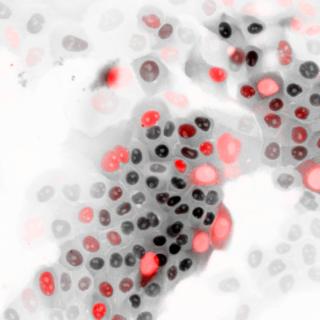
Enzymatic activity during the start of the cell cycle could be harnessed to inhibit cancer cell reproduction.
Read MoreCancer Immunology Data Engine uncovers secreted proteins with therapeutic potential
CCR researchers have developed a new tool that identifies secreted proteins associated with immunotherapy outcomes.
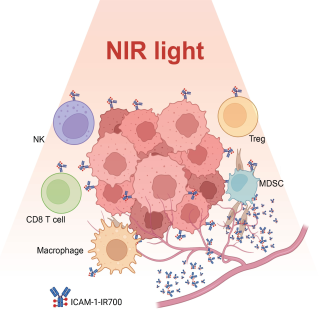
Read MoreCCR-developed cancer therapy shrinks tumors and boosts immune response
A targeted cancer therapy developed at CCR shows promise in its ability to target a common cancer cell marker, both shrinking tumors and producing a boosted immune response.
Read MoreClinical trial researching drug therapy for colorectal cancer
A clinical trial led by Tim F. Greten, M.D., Deputy Chief and Senior Investigator in the Thoracic and GI Malignancies Branch, is researching a two-drug therapy for adults with metastatic colorectal cancer.
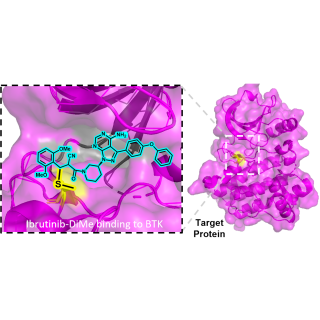
Read MoreSynthesized chemical component helps cancer drugs remain stable in water
Researchers developed a new chemical component that can be attached to drug molecules to make them more stable in water without losing effectiveness. This innovation could improve the performance of some cancer treatments.
Read MoreClinical trial researching immunotherapy for mesothelioma
A clinical trial led by Raffit Hassan, M.D., Chief and Senior Investigator in the Thoracic and GI Malignancies Branch, is researching an immunotherapy treatment for adults with solid tumors, including mesothelioma.
Read MoreClinical trial researching drug therapy for multiple myeloma
A clinical trial led by Elizabeth M. Hill, M.D., Assistant Research Physician in the Lymphoid Malignancies Branch, is researching a combination drug therapy for myeloid malignancies.
Read MoreCombining bevacizumab with erlotinib shrinks tumors in patients with rare and aggressive kidney cancer
Seventy-two percent of patients with HLRCC-associated kidney cancers, for which there previously was no known drug treatment, responded to the therapy in a clinical trial.
Read More