Clinical Trials
Combination therapy for solid tumors and small-cell cancers studied in new clinical trial
A clinical trial of a drug combination to treat solid tumors and small-cell cancers is being conducted at the NIH Clinical Center. PARP inhibitors can work better when combined with chemotherapy, such combinations can be too toxic, so this study uses a new kind of chemotherapy called PLX038 and combines it with a PARP inhibitor rucaparib to see if the combination of PLX038 and rucaparib can safely shrink solid tumors and small-cell cancers.
Read MoreClinical trial studies combination immunotherapy for colorectal cancer
Colorectal cancer (CRC) affects the colon and rectum, which are located at the lower end of the digestive tract. One of the most common cancers, it often spreads to the liver. Because treatments that aim to use the patient’s own immune system to attack mCRC have not been very successful so far, investigators are leading a study that combines two different types of immunotherapy to see if one can enhance the effect of the other.
Read MoreClinical trial evaluates a combination therapy for metastatic clear cell renal carcinoma
Kidney cancer is the 6th most common cancer diagnosed in the United States with greater than 73,000 new cases seen each year. CCR investigators are interested in studying a combination immunotherapy for its most common subtype, clear cell renal carcinoma, using Avelumab and interleukin (IL)-15.
Read MoreClinical trial evaluates immunotherapy for head and neck cancer caused by HPV
A clinical trial, led by Christian Hinrichs, studies neoadjuvant immunotherapy for HPV-related head and neck cancer. Neoadjuvant means it is given before main treatments such surgery. The goal of the study is to see if T cells given before the main treatment can reduce the risk of the disease coming back and to convert borderline or unresectable tumors to resectable.
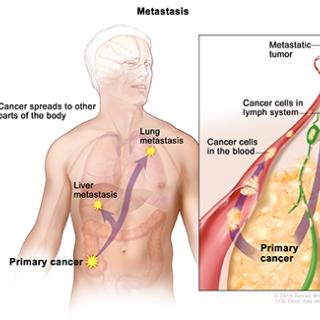
Read MoreTrial opens to evaluate a potential anti-metastasis compound
The Center for Cancer Research has opened a phase I trial to evaluate the safety and clinical activity of metarrestin, a compound that suppressed metastasis and extended survival in several preclinical cancer models.
Read MoreClinical trial will evaluate chemotherapy-sparing approach to treating Kaposi sarcoma
CCR physician-scientists have launched a clinical trial to evaluate whether the immune-stimulating molecule NHS-IL12, alone or in combination with an experimental immunotherapy called M7824, reduces tumors in patients with Kaposi sarcoma.
Read MoreFDA grants rare pediatric disease designation for experimental immunotherapy for acute lymphoblastic leukemia
A cell-based immunotherapy that is currently being evaluated at the NIH Clinical Center for the treatment of B-cell acute lymphoblastic leukemia (ALL) has been designated by the U.S. Food and Drug Administration as a drug for a rare pediatric disease. Designation could encourage development of this novel therapy for children with relapsed or refractory ALL.
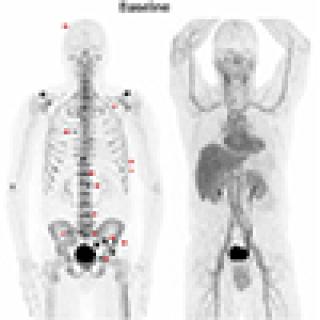
Read MoreClinical trial evaluates radiopharmaceutical as therapy for biochemically recurrent prostate cancer
Some men who have been treated for localized prostate cancer (PC) with surgery or radiation still have signs of the disease that are only detected by a blood test (a rising prostate specific antigen or PSA). This is called biochemically recurrent prostate cancer (BCRpc). Investigators in the Center for Cancer Research are leading a clinical trial exploring an option meant to be less toxic for treating BCRpc, which may impact microscopic bone disease seen only on PET scans, using radium-223.
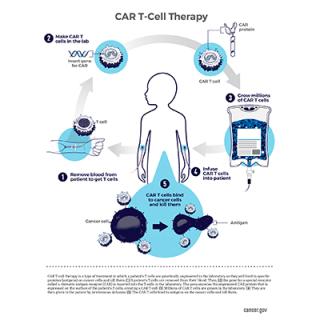
Read MoreImmunotherapy clinical trial tests therapy for metastatic solid tumors
Tumor-infiltrating lymphocytes (TILs) are white blood cells (T cells) that have moved from the blood into a tumor. While tumor cells frequently change their molecular structure to avoid attack by the immune system’s T cells, recent studies by the Center for Cancer Research’s Surgery Branch have found that most TILs don’t recognize newly mutated tumor cells. A clinical trial led by Steve Rosenberg, M.D., Ph.D., engineers T cells to recognize newly altered cancer cells that are then given back to the patient to help the immune system kill cancer cells in solid tumors.
Read MoreTwo patient’s stomachs kept “alive” after removal in novel study to understand stomach cancer
Two women with genetic predisposition to stomach cancer participated in a clinical trial at the Center for Cancer Research where their stomachs were removed and kept “alive” for several days, allowing the researchers to study the development of cancer and the effects of different therapies in unprecedented detail. The goal is to better study stomach cancer under realistic conditions and find novel, effective treatments.
Read More