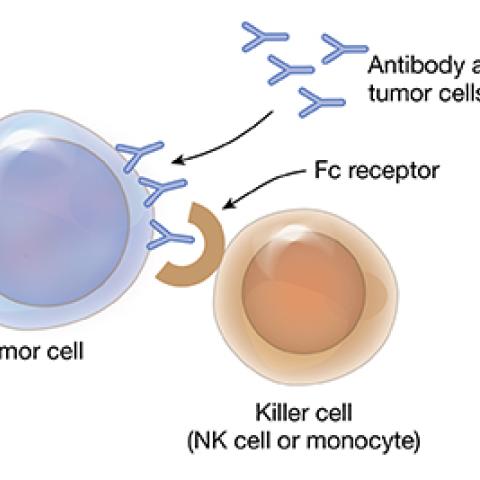
Antibody-dependent cell-mediated cytotoxicity
Image courtesy of Milos Miljkovic
Patients with certain mature T-cell cancers that have not responded to prior treatment may be eligible to participate in a clinical trial at the NIH Clinical Center.
Adult T-cell leukemia/lymphoma (ATLL) and mycosis fungoides/Sézary syndrome (MF/SS) are rare, fast-growing cancers that form in T cells, a type of white blood cell that is key to the body’s immune response. ATLL is found in the blood while MF/SS is a non-melanoma skin cancer. ATLL and MF/SS are known as mature T-cell cancers because they start in T cells that have matured in the thymus gland and then spread to other sites in the body. ATLL and MF/SS do not respond well to standard cancer treatments. Milos Miljkovic, M.D., M.Sc., Assistant Research Physician in the Lymphoid Malignancies Branch, is testing a combination therapy for patients whose ATLL and MF/SS has returned after therapy or has not responded to therapy. Mogamulizumab targets and blocks a receptor that is found on most ATLL and MF/SS cells. It is given to patients through a vein in the arm. Patients also receive intravenous interleukin-15 (IL-15), a protein that signals many types of immune cells to multiply and attack cancer cells. Investigators want to see whether IL-15 can enhance the anticancer effect of mogamulizumab and determine the safety and best dose of intravenous mogamulizumab and IL-15 for patients with ATLL and MF/SS.
Clinicaltrials.gov identifier: NCI-20-C-0011
NCI Protocol ID: NCT04185220
Official Title: Phase 1 Study of Recombinant Human IL-15 (rhIL-15) and Mogamulizumab for Patients With Refractory or Relapsed Adult T-Cell Leukemia and Mycosis Fungoides/Sezary Syndrome
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.