Clinical Trials
Joe Chinquee volunteers with Navajo Nation during COVID-19 pandemic
Joe Chinquee, D.H.Sc., M.B.A., M.T. (A.S.C.P.) D.L.M., Clinical and Scientific Manager of the Laboratory of Pathology, served on the frontlines of the COVID-19 pandemic. In an effort to give back, he was deployed on a month-long mission to assist the U.S. Public Health Service at the Navajo Nation in New Mexico. Chinquee said: “At the NIH, I’ve been given so many opportunities and rewards. I will never stop giving back, but I would not be able to volunteer for these missions without the full support of my NCI leadership.”
Read MoreLieutenant and Research Nurse Matt Lindsley shares his experiences and public service role
Matthew Lindsley, MPH, MSN, RN PHNA-BC, is a Lieutenant in the United States Public Health Service. As a Research Nurse Specialist for the NCI’s Center for Cancer Research, Neuro-Oncology Branch, Matt helps patients with brain and spine tumors who come to NIH for treatment. He was recently deployed to aid in the COVID-19 response mission in Washington state for a number of weeks.
Read MoreClinical trial tests combination therapy for glioblastoma multiforme
Glioblastoma multiforme (GBM) is a type of brain cancer where treatments include radiation therapy, chemotherapy and surgery, but survival rates are poor. Investigators are testing an anticancer drug selinexor, which may make GBM cells less resistant to radiation therapy and allow radiation therapy to kill more cancer cells.
Read MoreTrial shows aggressive cancer treatment is appropriate for people with HIV-associated primary central nervous system lymphoma
New research from CCR scientists shows that chemoimmunotherapy with antiretroviral therapy can lead to long-lasting remissions of HIV-associated primary central nervous system lymphoma without compromising neurocognitive function.
Read MoreClinical trial studies combination therapy for certain mature T-cell cancers that have not responded to treatment
Adult T-cell leukemia/lymphoma (ATLL) and mycosis fungoides/Sézary syndrome (MF/SS) are fast-growing cancers that form in T cells, a type of white blood cell that is key to the body’s immune response. A clinical trial is being conducted at the NIH Clinical Center to test a combination therapy for these rare cancers.
Read MoreClinical trial tests targeted radioactive agent as therapy for metastatic prostate cancer
Prostate-specific membrane antigen (PSMA) is present on the surface of all prostate cancer cells but is highly expressed on cells of castration-resistant prostate cancer (mCRPC). Frank Lin, M.D., Investigator in the the Molecular Imaging Program, is leading NCI’s effort to study the effect of targeted treatment with a radioactive agent to test dosing and overall effect for these patients.
Read MoreA Conversation with Christina Annunziata, M.D., Ph.D.
Christina Annunziata, M.D., Ph.D., is an Investigator in the Women’s Malignancies Branch at the Center for Cancer Research. She has spent most of her career studying the molecular underpinnings of ovarian cancer with the goal of discovering novel treatments. In our Q&A, Dr. Annunziata discusses her personal and professional milestones as well as new directions for her research.
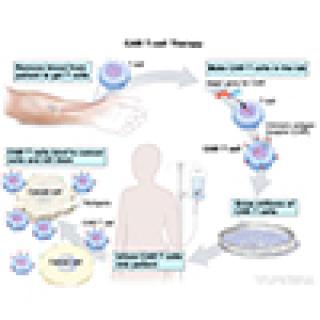
Read MoreClinical trial studies CAR T-cell therapy for relapsed/refractory acute myeloid leukemia
Acute myeloid leukemia (AML) is a cancer of the blood and bone marrow, the soft inner part of long bones where new blood cells are made. With AML, bone marrow cells don't grow the way they're supposed to. Instead, immature bone marrow cells grow uncontrollably and build up in the body. This study is testing the effect of chimeric antigen receptor T cells (CAR T cells) for children and young adults.
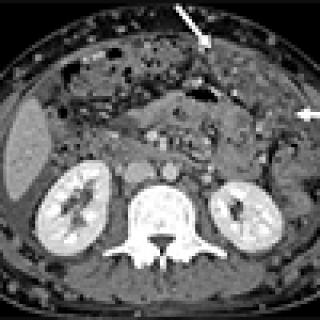
Read MoreNew clinical trial evaluates chemotherapy delivery for stomach cancer
A clinical trial testing chemotherapy delivery for stomach cancer that has spread to the lining of abdominal cavity is underway at the NIH Clinical Center. Investigators are evaluating how this delivery may improve outcomes for patients with this rare condition.
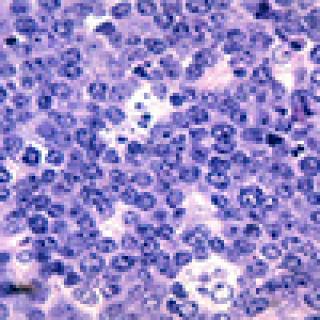
Read MoreStudy confirms effective, less toxic alternative to standard treatment for adults with Burkitt lymphoma
In a new study, an alternative treatment regimen that is less toxic than standard dose-intensive chemotherapy was found to be highly effective for adults with Burkitt lymphoma across all age groups and independent of HIV status. In addition to being better tolerated, the regimen, called dose-adjusted (DA) EPOCH-R, is already an option for diffuse large B-cell lymphomas and can be administered in an outpatient setting. The study was led by researchers in the Center for Cancer Research, and the DA-EPOCH-R regimen was originally developed by researchers led by Wyndham Wilson, M.D., Ph.D., Senior Investigator in the Lymphoid Malignancies Branch.
Read More