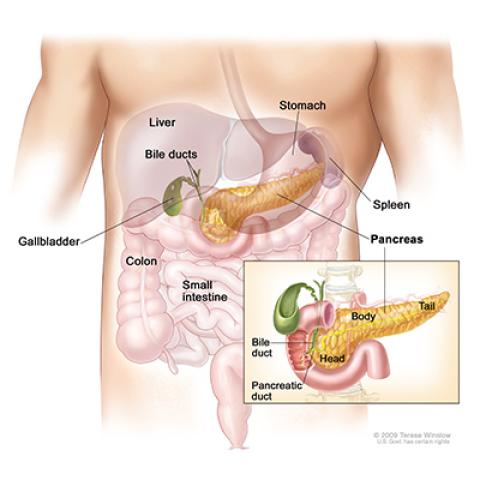
Anatomy of the pancreas.
Image courtesy of NCI Visuals Online
Patients with previously treated cancer of the pancreas or bile ducts or other mesothelin-expressing solid tumors may be eligible to participate in a new clinical trial at the NIH Clinical Center.
Mesothelin is a protein found on the surface of certain types of normal cells and cancer cells. It may help cells stick together and send signals. Some cancer cells, such as those of the pancreas and bile ducts, express higher-than-normal amounts of mesothelin. Christine Alewine, M.D., Ph.D., of the Laboratory of Molecular Biology, is leading a study that uses LMB-100 combined with a drug commonly used to treat certain types of arthritis and colitis. In some studies, this drug prevented antidrug antibodies (ADAs) from forming. LMB-100 is a man-made protein that is attracted to mesothelin. In previous studies, after binding to mesothelin on tumors, LMB-100 attacked and killed cancer cells. However, after initial treatment with LMB-100 many patients formed antidrug antibodies (ADAs) that neutralized the effect of subsequent injections of the drug. Investigators want to see if giving this combination therapy can prevent or delay the formation of ADAs, allowing patients to receive additional effective doses of LMB-100.
Clinicaltrials.gov identifier: NCT04034238
NCI Protocol ID: NCI-19-C-0128
Official Title: A Phase I Study of Mesothelin-Targeted Immunotoxin LMB-100 in Combination With Tofacitinib in Persons With Previously Treated Pancreatic Adenocarcinoma, Cholangiocarcinoma and Other Mesothelin Expressing Solid Tumors
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.