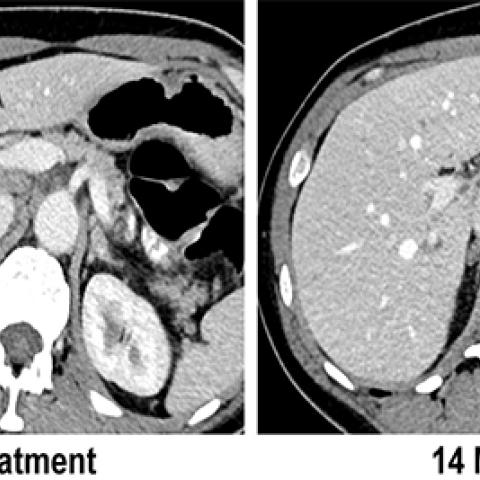
Before and after CT scan images of the liver of a patient with metastatic breast cancer (arrows) who was treated with T cells that recognized mutations in her tumor.
Photo courtesy of Steve Rosenberg
People 18–70 years old with metastatic solid tumors that have not responded to standard treatments may be eligible to participate in a clinical trial at the NIH Clinical Center.
Some people have solid tumors that have spread, or metastasized, and have not responded to standard treatments. Steven A. Rosenberg, M.D., Ph.D., Chief of the Surgery Branch, is leading a study of a new treatment for these cancer patients. Tumor-infiltrating lymphocytes (TILs) are white blood cells (T cells) that have moved from the blood into a tumor. Tumor cells frequently change their molecular structure to avoid attack by the immune system’s T cells. Recent studies by the Surgery Branch have found that most TILs don’t recognize newly mutated tumor cells. However, a very small number of TILs do recognize recent mutations in certain proteins within tumor cells and are able to attack these cells. The scientists in the Surgery Branch can extract information from that very small number of cells and genetically engineer a larger number of T cells to recognize the mutations. Patients in this study will undergo leukapheresis, where a small tube in one arm will remove blood and send it to a machine that will separate white blood cells from the rest of the blood. The blood will then be returned to the patient through a tube in the other arm. In a laboratory, the special T cells that can recognize newly altered cancer cells will be grown in large numbers then given back to the patient to help the immune system kill cancer cells. Investigators want to see if this gene transfer therapy can shrink solid tumors for patients with non-small cell lung cancer, breast cancer, gastrointestinal/genitourinary cancer or ovarian cancer.
Clinicaltrials.gov identifier: NCT03412877
NCI Protocol ID: NCI-18-C-0049
Official Title: Administration of Autologous T-Cells Genetically Engineered to Express T-Cell Receptors Reactive Against Mutated Neoantigens in People With Metastatic Cancer
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.


