Learn about our mission, research interests and goals, and organization.
Center for Immuno-Oncology
Center for Immuno-Oncology
The CCR’s Center for Immuno-oncology (CIO) explores fundamental questions of cancer immunotherapy through rigorous preclinical studies and translates these findings into clinical trials with the goal of developing novel therapies for a spectrum of cancers.
CCR’s long history of excellence in immunology has contributed to advances in immunotherapies now widely used to treat many patients who have cancer. The CIO acts as a hub for CCR’s many immuno-oncology efforts to further promote and support these efforts and build on the existing expertise, serving as a nexus for multidisciplinary and collaborative efforts among investigators in multiple laboratories, branches and programs across CCR, NCI, and NIH, as well as with investigators in the academic community and the private sector.
Clinical Trials
PI & Key Staff
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
News
Training
NCI Immunotherapy Fellowship Overview
This training program is designed for physicians who have completed a medical oncology fellowship program in the United States and who seek specialized training in immunotherapy with clinical trials and clinical trial development.
There are multiple opportunities for training in clinical immunotherapy at the Center for Cancer Research (CCR), National Cancer Institute (NCI), with active clinical programs in therapeutic cancer vaccines, immune checkpoint modulation, adoptive cellular therapies (both TCR transgenic and CAR-T therapy) and antibody-based immunotherapies. This training program, at the CCR in Bethesda, Maryland, allows the fellow to have exposure to multiple clinical immunotherapeutic approaches and also to key opinion leaders in the field of clinical immunotherapy. This 1-year fellowship provides opportunities to understand how to design, write and run clinical trials, how to treat patients, how manage toxicities, as well as opportunities to work with multiple experimental agents.
Funding and Co-Sponsorship
This fellowship is co-sponsored by the National Cancer Institute and the Society for Immunotherapy of Cancer (SITC) and made possible, in part, by an educational grant from EMD-Serono.
Surgery Branch / Cancer Immunotherapy Research Fellowship
This training program is for general surgery residents who have completed at least two years of an ACGME accredited general surgery residency. The program provides training in Surgical Oncology, an immersive experience in immunotherapy laboratory research, and clinical human research in early phase trials.
Gallery
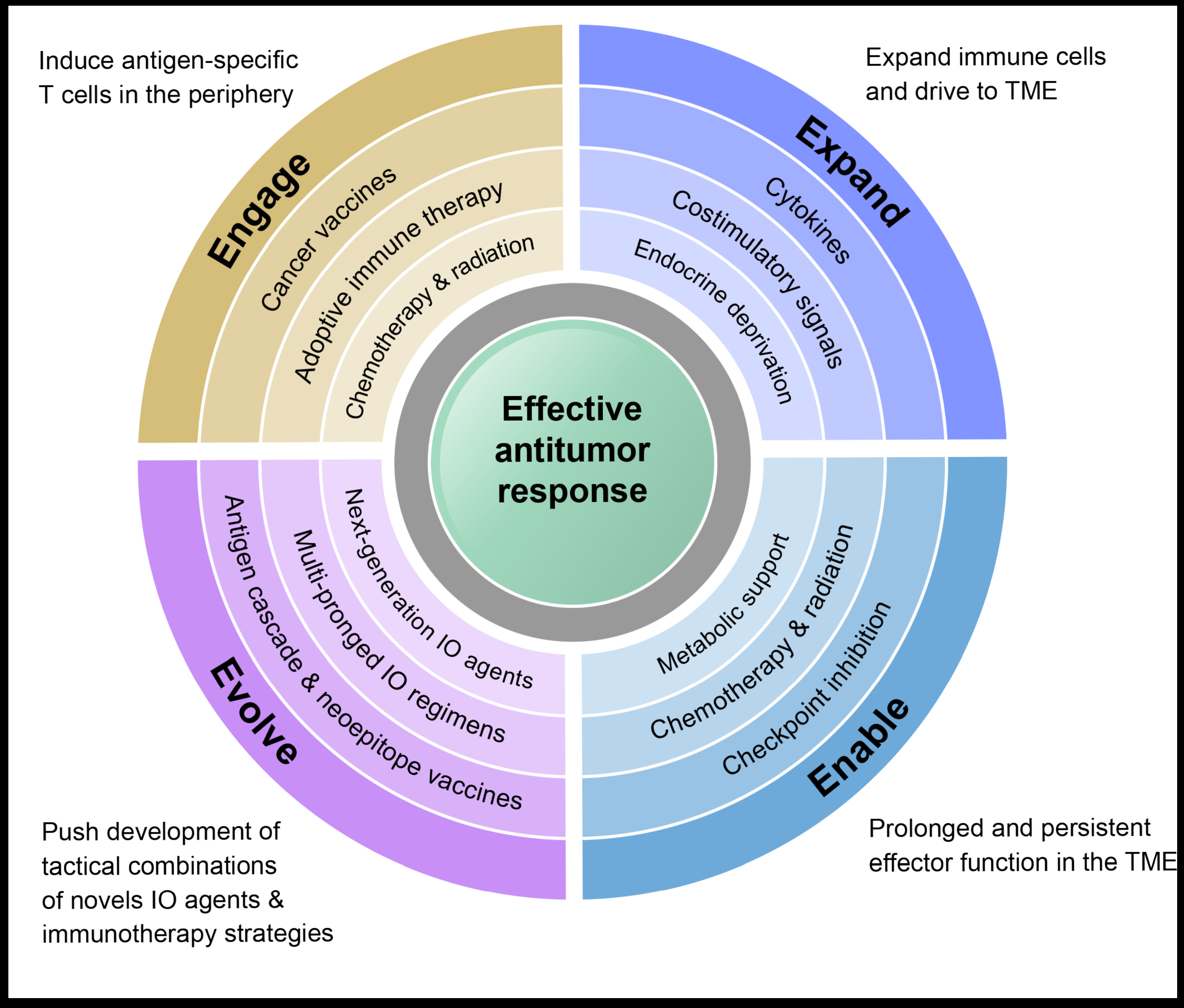
Multimodal immunotherapy to Engage, Expand, Enable, and Evolve anti-tumor immune responses.
Fabian, et al. Differential combination immunotherapy requirements for inflamed (warm) tumors versus T cell excluded (cool) tumors: engage, expand, enable, and evolve. J Immunother Cancer. 2021;9(2):e001691.
Seminars
The CIO Distinguished Lecture Series is a premier virtual seminar series aimed at showcasing groundbreaking advancements in immunotherapy for cancer research. Recent highlights include clinical successes in CAR-T cell therapies targeting solid tumors, translational breakthroughs in bispecific T-cell engagers (BiTEs) for enhanced tumor specificity, and bench research on novel immune checkpoint inhibitor combinations that overcome key resistance mechanisms. The series also explores the development of personalized neoantigen vaccines and strategies for modulating the tumor microenvironment to boost immune response. By fostering a collaborative environment, the series disseminates cutting-edge research findings across clinical, translational, and bench research, bridging the translation of novel immunotherapeutic strategies into clinical practice and ultimately advancing our understanding and treatment of various cancers. The audience are scientists and clinical researchers within Center for Immuno-Oncology at the National Cancer Institute (NCI); however, we often accrue upwards of 200 attendees from across the NCI keen on gaining valuable insights into the latest scientific discoveries and technological innovations helping to shape the future of cancer immunotherapy.
CIO Distinguished Lecture Series Speakers include:
2022: Drs. Lisa Butterfield, Robert Ferris, Marcella Maus, Greg Delgoffe, Sandra Demaria, Jeffrey Miller, and William Murphy
2023: Drs. Robert Herman Vonderheid, Michael Caligiuri, Christopher Klebanoff, Elisabeth de Vries, Tak Mak, Saman MalekiVreki, Jeffrey Miller, and Cassian Yee
2024: Drs. Bernard Fox, Thomas Marron, Lawrence Fong, Saul Priceman, Jennifer Guerriero, Catherine Wu, and Franco Marincola