News and Events
New Strategy Shows Promise Against Deadly Breast Cancer in the Brain
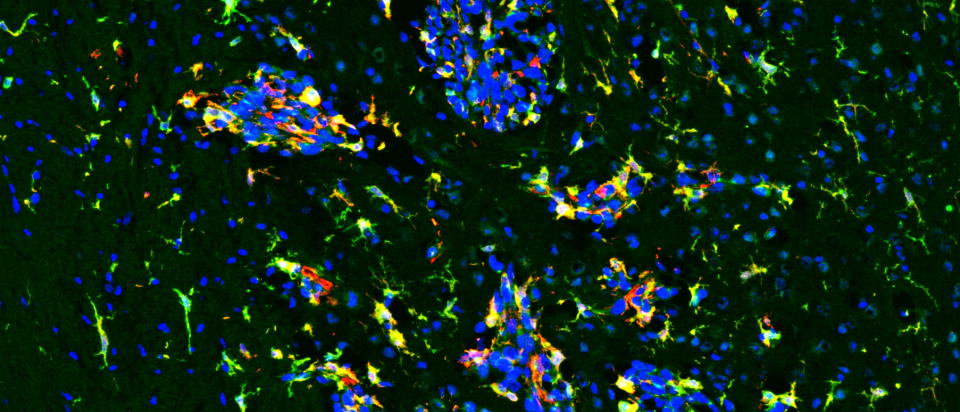
A new NIH study points to a promising strategy for treating aggressive breast cancer that spreads to the brain, a complication with few effective options. Learn how blocking a key brain cell survival pathway could open the door to future therapies.
Read MoreSung-Yun Pai selected for Hedwig van Ameringen Executive Leadership in Academic Medicine® (ELAM) Program
Sung-Yun Pai, M.D., Chief of the Immune Deficiency Cellular Therapy Program, was selected for the Hedwig van Ameringen Executive Leadership in Academic Medicine® (ELAM) Program for Women at Drexel University College of Medicine. The ELAM® program is dedicated to developing leadership and management skills in healthcare and is designed for senior-level women leaders interested in advancing hospitals and healthcare systems.
Read MoreCelebrating Asian American, Native Hawaiian, and Pacific Islander Heritage Month: A Conversation with Lilian Yang, M.B.A.
Lilian Yang, M.B.A., is the Senior Branch Manager in the Neuro-Oncology Branch (NOB). She is originally from Hawaii and identifies as Chinese American. In this Asian American, Native Hawaiian, and Pacific Islander Heritage Month Q&A, she talks to us about the many places she has lived, some valuable business school advice, and the need for Asian representation in leadership roles.
Read MoreCelebrating AANHPI Heritage Month: A Conversation with Suresh Ambudkar, Ph.D.
Suresh V. Ambudkar, Ph.D., is a Senior Investigator and the Deputy Chief of the Laboratory of Cell Biology. For this Asian American, Native Hawaiian, and Pacific Islander Heritage Month Q&A, he answers some questions about his academic, research and personal journey immigrating from India to the United States.
Read MoreClinical trial researching immunotherapy for B-cell lymphoma
A clinical trial led by Mark Roschewski, M.D., Senior Clinician in the Lymphoid Malignancies Branch, is researching immunotherapy for indolent B-cell lymphoma, a cancer of white blood cells that frequently relapses.
Read MoreMachine learning reveals rules of T cell activation
CCR and McGill University scientists have used machine learning to build models that can predict T cell responses against tumors.
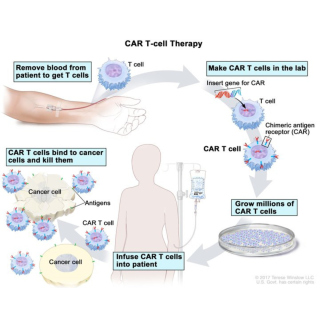
Read MoreClinical trial researches CAR T-cell therapy for hairy cell leukemia
Robert J. Kreitman, M.D., Senior Investigator in the Laboratory of Molecular Biology, is leading a study to see whether CAR T-cell therapy impacts patients with hairy cell leukemia.
Read MoreClinical trials research immunotherapy for Kaposi sarcoma
Clinical trials led by Ramya Ramaswami, M.B.B.S., M.P.H., Physician-Scientist Early Investigator in the HIV and AIDS Malignancy Branch, are researching immunotherapy for Kaposi sarcoma.
Read MoreIn Memoriam: Joost J. Oppenheim, M.D. (1934–2022)
The CCR mourns the recent passing of colleague and friend Joost Oppenheim, M.D., Senior Investigator and Head of the Cellular Immunology Section in the Cancer Innovation Laboratory.
Read MoreFour CCR researchers receive high honors
CCR Senior Investigators Michael Lichten, Ph.D., Deborah Morrison, Ph.D., Mary Carrington, Ph.D, and Susan Lea, D.Phil., F.Med.Sci., have been elected to notable scientific academies and societies in recognition of their significant and impactful research accomplishments.
Read MoreElaine S. Jaffe receives HHS Career Achievement Award
Elaine S. Jaffe, M.D., NIH Distinguished Investigator in the Laboratory of Pathology, was awarded a 2022 Career Achievement Award by the Department of Health and Human Services in recognition of her groundbreaking advances in the diagnosis and treatment of patients with blood cancers.
Read More