News and Events
Proteins Released from the Nuclei of Dying Cancer Cells Promote Tumor Growth
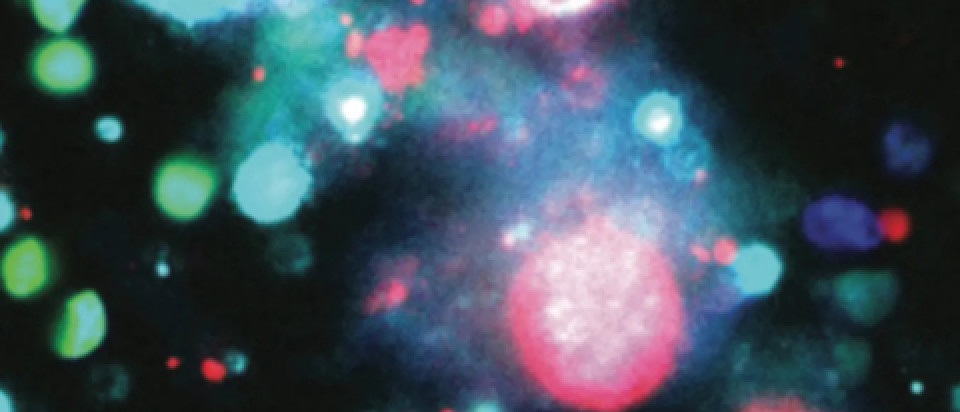
Material released from dying cancer cells, known as tumor cell nuclear expulsion products (TuNEPs), contains specific proteins that promote the growth of neighboring cancer cells. Targeting these proteins could lead to new treatments that hinder cancer spread and improve patient outcomes.
Read MoreCCR presentations at AACR 2020 - Session II
The American Association for Cancer Research (AACR) Annual Meeting covers the latest discoveries across the spectrum of cancer research—from population science and prevention; to cancer biology, translational, and clinical studies; to survivorship and advocacy—and highlights the work of the best minds in research and medicine from institutions all over the world. The 2020 AACR Virtual Annual Meetings I and II will take place April 27-28 and June 22-24, respectively. View the list of CCR presenters for the June meeting.
Read MoreCCR scientists develop new blood test that may improve liver cancer screening
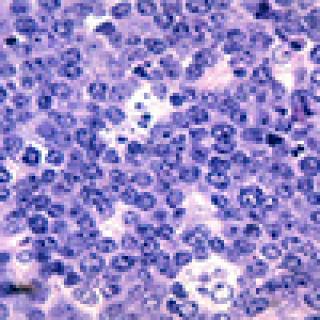
Scientists led by Xin Wei Wang, Ph.D., Deputy Chief of the Laboratory of Human Carcinogenesis, have developed a new test that can identify people who are likely to develop hepatocellular carcinoma (HCC), the most common form of liver cancer. The approach uses a simple blood test to check for the patient’s previous exposure to certain viruses. “Together with existing screening tests, the new test could play an important role in screening people who are at risk for developing HCC. It could help doctors find and treat HCC early. The method is relatively simple and inexpensive, and it only requires a small amount of blood,” he says.
Read MoreIn Memoriam: Isaiah Fidler, D.V.M, Ph.D.
The Center for Cancer Research mourns the recent death of past colleague and friend Isaiah Fidler, D.V.M., Ph.D. He joined the National Cancer Institute in 1975 and led the metastasis program at the Frederick Cancer Research Facility. His eight years at NCI produced some of his early innovative work in unraveling the riddles of how cancer spreads.
Read MoreA Conversation with Bríd Ryan, Ph.D., M.P.H.
Bríd Ryan, Ph.D., M.P.H., is an NIH Stadtman Investigator in the Laboratory of Human Carcinogenesis at the Center for Cancer Research. Her research focuses on health disparities related to lung cancer across different populations. She discusses her latest research findings and future directions of her work as well as the importance of the supportive CCR environment in this Q&A.
Read MoreStudy confirms effective, less toxic alternative to standard treatment for adults with Burkitt lymphoma
In a new study, an alternative treatment regimen that is less toxic than standard dose-intensive chemotherapy was found to be highly effective for adults with Burkitt lymphoma across all age groups and independent of HIV status. In addition to being better tolerated, the regimen, called dose-adjusted (DA) EPOCH-R, is already an option for diffuse large B-cell lymphomas and can be administered in an outpatient setting. The study was led by researchers in the Center for Cancer Research, and the DA-EPOCH-R regimen was originally developed by researchers led by Wyndham Wilson, M.D., Ph.D., Senior Investigator in the Lymphoid Malignancies Branch.
Read MoreFDA approves pomalidomide for AIDS-related Kaposi sarcoma
On May 14, 2020, the Food and Drug Administration expanded the indication of pomalidomide (POMALYST, Celgene Corporation) to include treating adult patients with AIDS-related Kaposi sarcoma after failure of highly active antiretroviral therapy and Kaposi sarcoma in adult patients who are HIV-negative. This oral therapy is the first new treatment option available for those with Kaposi sarcoma in more than 20 years.
Read MoreIn Memoriam: Stephen Oroszlan, Ph.D.
The Center for Cancer Research mourns the recent death of past colleague and friend Stephen Oroszlan. He was an esteemed member of the NCI community from 1976-1995 and served as a Scientist Emeritus since 1995.
Read MoreEytan Ruppin quoted in New York Times story on treating COVID-19
The New York Times has reported on a new paper detailing drugs with the potential to treat COVID-19 by researchers from international institutions. Eytan Ruppin, Ph.D., Chief of the Cancer Data Science Laboratory, turned the computer network his lab uses toward analyzing some of the drugs that did best in the screening to predict which are likely to succeed in actual patients and in what combinations.
Read MoreIra Pastan selected as a 2020 Sammies finalist
Ira Pastan, M.D., Co-Chief of the Laboratory of Molecular Biology, has been selected as one of 27 Samuel J. Heyman Service to America Medal (Sammies) finalists. These finalists are outstanding federal employees who serve the public good and are addressing many of our country’s greatest challenges. Dr. Pastan is nominated for discovering a new class of drugs that can successfully treat a rare form of leukemia and hold promise to be effective therapies for pancreatic and lung cancer as well as mesothelioma.
Read MoreJohn Schiller elected to the National Academy of Sciences
John T. Schiller, Ph.D., Deputy Chief of the Laboratory of Cellular Oncology, has been elected to the National Academy of Sciences for his distinguished and continuing achievements in original research. Dr. Schiller, in close partnership with Dr. Doug Lowy, has made fundamental contributions to our understanding of human papillomavirus (HPV) culminating in the development of the prophylactic vaccines Cervarix and Gardasil, which protect from HPV-related cancers, including cervical cancer.
Read More