News and Events
New Strategy Shows Promise Against Deadly Breast Cancer in the Brain
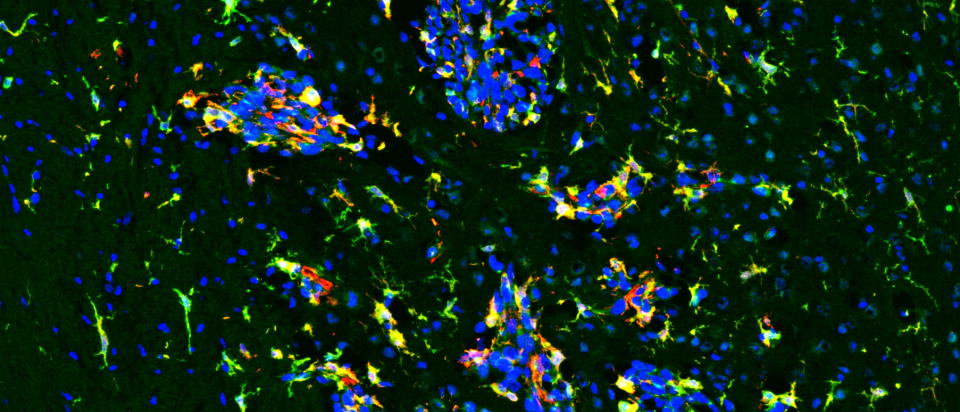
A new NIH study points to a promising strategy for treating aggressive breast cancer that spreads to the brain, a complication with few effective options. Learn how blocking a key brain cell survival pathway could open the door to future therapies.
Read MoreDrug lessens symptoms of severe chronic graft-versus-host disease
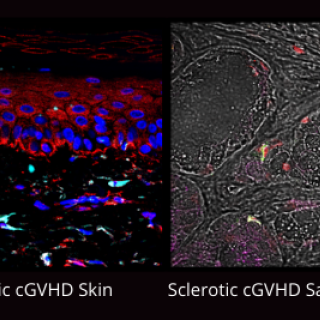
About half of patients who receive allogeneic stem cell transplants, a treatment for blood cancer, develop a difficult-to-treat condition called chronic graft-versus-host disease (cGVHD). CCR investigators have found that pomalidomide, an immune-modulating drug, can reduce symptoms in patients with severe cases of cGVHD.
Read MoreA key mechanism that fuels uncontrolled cell growth is uncovered in yeast
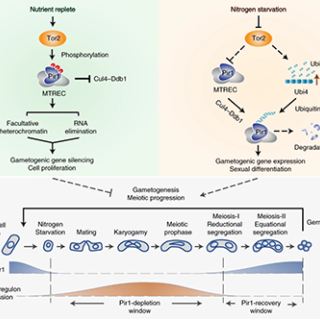
Scientists have long wondered how cancer cells use a protein complex, called TOR, to survive and proliferate in nutrient-poor conditions. Now, CCR researchers have discovered how a protein that is targeted by TOR drives this process, which holds important implications for understanding cancer and some genetic disorders.
Read MoreRegistration and abstract submission now open for RNA Biology Symposium
The symposium offers the opportunity to learn more about the current status of RNA biology in development and disease, share research with leading figures in the field and discuss the use and implications of these advances for clinical applications. Sessions Include: RNA Processing; RNA Structure and Mechanism; Non-Classical RNAs; and RNA Therapy. Learn more...
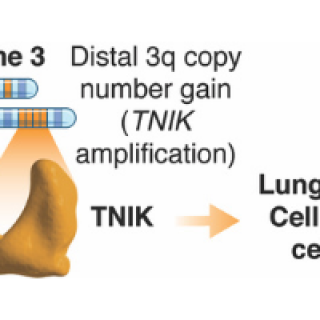
Read MorePotential therapeutic target for lung squamous cell carcinoma identified
CCR researchers have identified the protein TNIK as a therapeutic target for lung squamous cell carcinoma, the second most common type of lung cancer. Using human lung cancer cells transplanted into preclinical models, researchers found that the cells responded to a pharmacological treatment that inhibited TNIK and also resulted in cell death in the transplanted tumor cells.
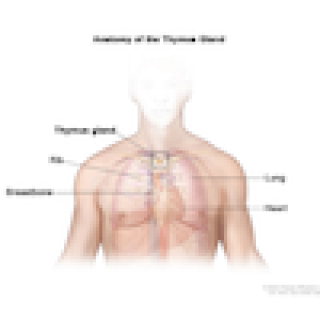
Read MoreClinical trial studies immunotherapy combination for recurrent thymoma and thymic carcinoma
Thymic epithelial tumors (TETs) are rare cancers that originate from the cells of the thymus, a small organ that sits in the chest between the lungs and above the heart. Arun Rajan, M.D., Senior Clinician in the Thoracic and GI Malignancies Branch, is leading a clinical trial of a therapy for thymoma and thymic carcinoma that has returned after chemotherapy and cannot be treated with surgery.
Read MoreFecal microbiota transplants help patients with advanced melanoma respond to immunotherapy
A collaborative study between the National Cancer Institute (NCI) and the UPMC Hillman Cancer Center at the University of Pittsburgh suggests that fecal microbiota transplants can help patients with advanced melanoma respond to immunotherapy. “Our study is one of the first to demonstrate in patients that altering the composition of the gut microbiome can improve the response to immunotherapy,” says study co-leader Giorgio Trinchieri, M.D., Chief of CCR’s Laboratory of Integrative Cancer Immunology.
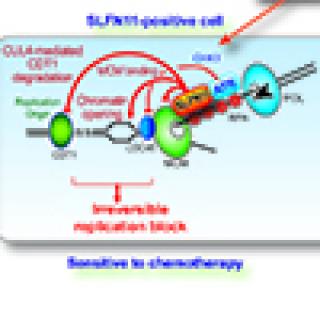
Read MoreNew way to address chemoresistance linked to the protein SLFN11
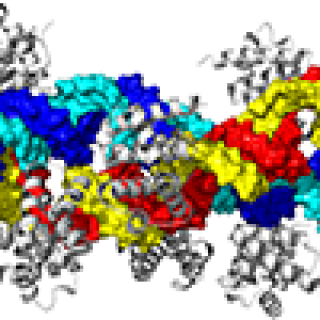
CCR researchers have discovered two complementary roles for the protein Schlafen-11 (SLFN11) in determining patient response to chemotherapy. These findings have implications for how to overcome this resistance and provide new treatment options for patients with small cell lung cancer (SCLC) and many other cancers.
Read MoreGenetics, not just smoking, influence small cell lung cancer risk
People with mutations in DNA repair genes may be more likely than others to develop small cell lung cancer. Identifying these mutations in patients could help guide treatment decisions.
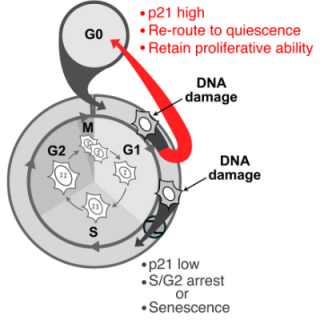
Read MoreNew findings show how damaged cells survive the cell cycle
As cells divide and replicate, important safety checkpoints are in place to ensure that most faulty cells with damaged DNA do not survive the cell cycle. In a new twist, CCR researchers discovered how some damaged cells use molecular inertia to drive past these safety checkpoints and continue through the cell cycle.
Read MoreNew insights into what fuels an aggressive form of kidney disease
Researchers have uncovered a key mechanism behind an aggressive form of kidney cancer, whereby cells lacking an important enzyme are unable to replicate and maintain healthy mitochondrial DNA. This results in more genetic abnormalities in the cells, fueling the growth and spread of cancer.
Read More